Abstract
Amnesia (memory loss) is a prevalent issue that occurs in invertebrates and vertebrates, which leads to forgetfulness. There are very limited treatment options available for this problem. Studies show that fish oil assists in improving the cognitive performance in humans with mild cognitive impairment. Fish oil contains omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). However, the precise role of fish oil and its effect on short term memory of an invertebrate species, D. melanogaster, is currently unknown. Here, we show how implementing fish oil into the diet of D. melanogaster impacts their short-term memory compared to fruit flies that are provided with a standard diet. We established that the fruit flies exposed to the fish oil diet learned faster than the fruit flies exposed to the standard diet. This suggests that fish oil can improve learning and memory in D. melanogaster. Our results demonstrate how adding fish oil to a fruit fly’s diet affects their memory. We anticipate this experiment to be a starting point in learning more about the role of polyunsaturated fatty acids (PUFAs) on learning and memory in humans with normal brain function.
Introduction
Memory loss is defined as forgetfulness over a period of time. According to a study, about 40% of people aged 65 or older have age-associated memory impairment in the United States (Small, 2002). There are two different types of memory loss: short term memory loss and long-term memory loss. Short term memory loss is when people are unable to remember recent details or activities that they have done in a short time period which is about 15 to 30 seconds while long term memory loss is when people are unable to remember details over long periods of time which can extend from a few minutes up to a lifetime (Atkinson and Shiffrin, 1968). Loss of memory is common and since memory is essential for an animal’s survival, loss of memory could pose several threats. There are several mechanisms leading to memory loss, but very little is known about the factors that could help prevent it (Jahn, 2013). Multiple factors play a role in affecting short- and long-term memory in humans. A few of these factors include age, environment, Alzheimer’s, dementia, and more (Plassman and Breitner, 1996). There are a few treatment options available for the diseases such as a good diet, a healthy lifestyle, and therapy for individuals, but they are very limited (Tsai, 2018).
Memory loss is most likely going to be different in vertebrates versus invertebrates. There are studies performed on memory loss in both vertebrates and invertebrates. These studies reveal that the genome in invertebrates is not as complex, suggesting fewer genes aiding in the learning response (Agranoff et al., 1999). Vertebrates have a more complex genome giving these species more of an advantage. The environment that an invertebrate is placed in goes hand in hand with their memory function in order to learn how to survive (Melillo et al., 2018). Therefore, if an environment is not at risk or does not pose a threat to an invertebrate’s survival, they are able to distinguish that and do not react. Invertebrates respond to what occurs in their environment in order to act accordingly (Melillo et al., 2018). An invertebrate species, D. melanogaster has been extensively studied (Pandey, 2011) in research and this has led to important discoveries. Its genome has already been sequenced and used to make different mutants (Kornberg, 2000). Nearly 75% of human disease-causing genes are believed to have a functional homolog in D. melanogaster (Pandey, 2011). Invertebrate models offer an advantage over vertebrate models because they provide a wide variety of experimental tools (Jeibmann and Paulus, 2009). Due to these advantages, memory in drosophila has been studied widely. There are several mechanisms leading to memory loss, however very little is known about the factors that could strengthen or help improve memory (Izquierdo, 1999).
Fish oil is known to help improve memory loss in adults with mild cognitive impairment (Storbel, 2009). For example, in one study, people aged 55 years or older with memory loss took fish oil supplements for six months to improve their short-term memory. They took a test that measured learning and memory skills and had nearly double the reduction in errors in comparison with the placebo (Storbel, 2009). In another study, 485 adults who had cognitive decline were given 900 mg of a placebo or DHA to improve their short-term memory. It was found that the group obtaining the DHA in the fish oil did better on tests relating to learning and memory (Yurko-Mauro et al,. 2010). Lastly, there was a study that showed that brain activity also improved short term memory when people took 1.8 grams of omega-3 fatty acids from fish oil for 24 weeks (Chiu et al., 2008).
Fish oil has several benefits including fetal development and reducing the risk of heart disease (Swanson et al., 2012). Additionally, fish oil combined with physical exercise in rats has shown to play a role in the enhancement of cognitive performance (Chung et al., 2008). While these studies reveal that fish oil has a positive effect on memory in humans with mild cognitive impairment, it is not clear if fish oil has an impact on memory in individuals with normal brain function. In the current study, we are testing our hypothesis that the short-term memory in D. melanogaster will be improved by supplementing their diet with fish oil. We predicted that flies provided with a fish-oil supplemented diet will learn faster than the control group provided with a standard cornmeal diet.
Materials & Methods
Fly Lines and Maintenance
20 wild type Oregon-R flies were obtained from Dr. Lee’s lab. The flies were cultured in a light/temperature-controlled incubator with a 12-hour light/dark cycle at 25 °C and were reared on a standard cornmeal diet (SD). Once the larvae were observed, the adult flies were separated and the larvae were allowed to grow. The new 2 to 3-week-old flies were divided into two groups and transferred to new vials. Group A consisted of 9 flies and was fed a standard cornmeal diet, while the diet for Group B consisting of 9 flies was supplemented with fish oil i.e; ~4 mg/mL EPA/DHA, (40% and 30% of each isomer, Dr. Tobias, Amazon). The flies were exposed to the respective diets for at least a week and were then used for the experiment. The flies were not separated based on sex for the experimental study.
In order to evaluate the feasibility of this experiment, a separate pilot study using 2 males and 4 females that were reared on a standard diet (SD) was conducted. Since sex difference is known to influence Drosophila’s behavior (Ali et al., 2011), the males and females were separated in our pilot study.
Phototaxis Test
Flies are naturally positively phototactic (Kain, 2012), meaning when given a choice between light and dark, they choose light more frequently (Le Bourg and Badia, 1995). However, a defect in the visual system of a fly or any other underlying problem could lead to changes in this behavior (Ali et al., 2011). Since this natural positive phototactic behavior of the flies was used to train them, each fly was examined for any defect that could change its behavior and hence necessitates its exclusion.
A t-maze was created in order to perform this test. The t-maze consisted of two chambers; a dark and light chamber, and a middle part with a trap door (Figure 1). It was constructed using two ~9.5 cm long vials. The vials were connected end to end in a horizontal position. The vial on the left was covered with aluminum foil to create a dark chamber. A light bulb was attached to the bottom of the right vial to create a light chamber. The middle part was used to connect the two vials and to place the trap door between them. The trap door, when closed, would prevent the flies in the dark chamber from entering the lighted chamber and vice versa.
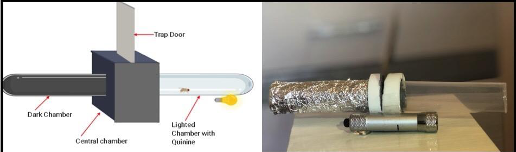
Figure 1: Design of t-maze and an image of actual t-maze
To test phototactic behavior, the flies were placed in the dark chamber with the trap door closed one at a time. The room lights were turned off and a red light was turned on. The red light was used because Drosophila’s visual system is known to be insensitive to the red light (Hanai, 2008). The flies were allowed to acclimatize to the dark chamber, with the red light on, for at least 10 seconds (Ali et al., 2011). After 10 seconds, the red light was turned off and a white light connected to the bottom of the light chamber of the t-maze was turned on. The trap door was opened and the two tubes were connected, allowing the flies to access the light chamber. The flies that entered the lighted chamber within 30 seconds of opening the trap door were considered to be positively phototactic and were used for memory training. The flies that did not enter the light chamber within 30 seconds of opening the trap door indicated defects in the visual system or some other underlying problem and were eliminated from the study. The flies were pre-selected through the phototaxis test because the phototactic ability of the flies was used to train them to associate light with an aversive stimulus in the APS assay.
Aversive Phototaxic Suppression Assay (APS)
The APS assay uses the positive phototactic behavior of flies to train them to associate the light with an aversive stimulus (Le Bourg, E., 2004). In order to train the flies and analyze the effect of fish oil on the memory of flies, the same t-maze was used however, this time a filter paper with the quinine solution was placed in the lighted chamber. The quinine hydrochloride dihydrate (CAS 6119-47-7, VWR) was used as an aversive stimulus. A 0.1 M solution was prepared by diluting 1.98 g of quinine hydrochloride with 50 mL of distilled water (Seugnet, 2009).
This experiment was performed on the 18 positively phototactic flies, 9 from group A, provided with the standard diet and 9 from group B, provided with the fish oil diet. One fly was trained at a time and the flies were starved for 6 hours before running this training session. The flies were placed one at a time in the dark chamber, with the trap door closed. The room lights were turned off and the red light was turned on. The flies were given 30 seconds to acclimatize to the dark chamber before starting the first trial. During this time, the walls of the lighted chamber were coated with 500µL of the originally prepared quinine solution using a filter paper. This amount was used based on the area and thickness of the filter paper. After 30 seconds, the light at the bottom of the light chamber was turned on, illuminating the light chamber and the trap door was opened. Since the flies were positively phototactic, they moved to the lighted chamber and tasted the bitter quinine, which acted as an aversive stimulus. After entering the light chamber, the flies were allowed to roam freely between the light and dark chamber for 1 minute, and then they were tapped back into the dark chamber. The trap door was then closed and the flies were allowed to rest for 10 seconds in the dark chamber. The trials were repeated for each fly and after entering the light chamber, the flies were given at least 1 minute to rest between successive trials. The number of trials conducted was different for each fly based on the time they took to learn.
To test the learning of flies from both groups, a test trial was conducted for each fly immediately after the training trials. After the light was turned on and the trap door was opened, the fly was given 250 seconds to move to the light chamber. Failure to move into the lighted chamber was considered a pass and the fly was considered to have learned to associate the lighted chamber with quinine. Failure to avoid the light chamber and associate it with the aversive stimulus was considered a failure.
Results
Before testing our main hypothesis stating that the fish-oil supplemented diet improves learning in D. melanogaster, we performed an experiment to see if our experimental set up was working as we intended (Pilot Study in Materials and Methods). The ANOVA test indicated that there were no statistically significant differences among the first four trials (Fig. 2a, p>0.05). Another ANOVA test was performed among the first five trials and significant differences were found (p<0.05). Then, a t-test was performed between the first and fifth trials, and it showed a significant difference between the first and fifth trials (Fig. 2a, p<0.05). This suggests that files start learning from the fifth trial. The upward trend in Figure 2a shows that the time taken by flies to enter the lighted chamber is increasing. In other words, the avoidance time increased with the number of trials. We interpreted that the flies had learned to associate the lighted chamber with quinine and were taking a significantly greater amount of time to enter the light chamber by the fifth trial. Results from our pilot study showed that there were no sex-based differences in learning (Fig. 2b, p>0.05). There was no statistically significant difference (p>0.05) in the time taken by males and females to learn (Fig. 2b).
When the flies in the standard diet (Group A) were compared to the flies in the fish oil diet (Group B), there was a statistically significant difference (p<0.05) in trials taken to learn. The fish oil group learned faster than the standard diet group (Fig. 2c), and flies in the fish oil group starting avoiding the lighted chamber earlier than the flies in the standard diet group. For this experiment (Fig. 2c), the flies were not separated based on sex.
When the learning test was conducted at the end of training trials, it was found that all the flies were able to avoid the lighted chamber. During the learning test, all of the flies took more than 250 seconds to enter the lighted chamber, suggesting that they have learned to associate light with an aversive stimulus (data not shown).
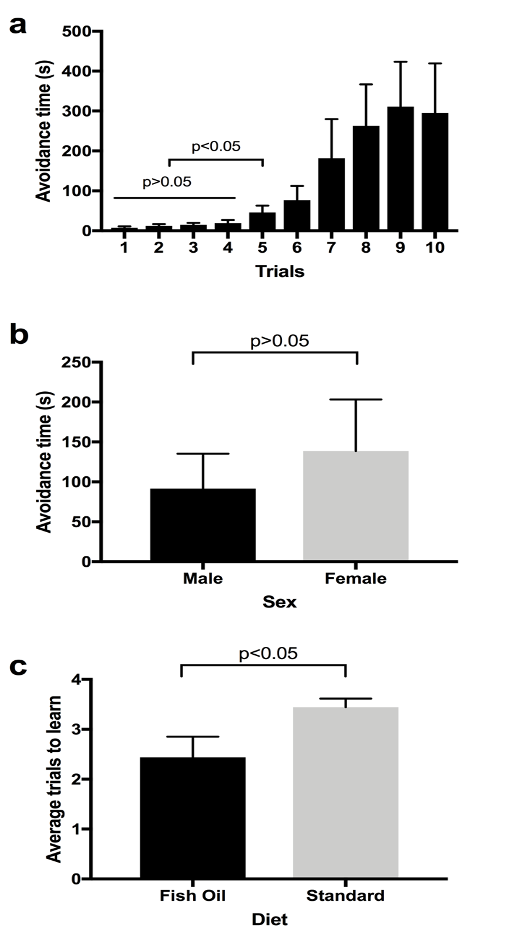
Figure 2. Aversive Phototaxic Suppression Assay. a) Avoidance time vs. number of trials (n=6, 4 females and 2 males). Avoidance time is the time taken by the flies to enter the lighted chamber. This figure shows average avoidance time by both male and female flies to enter the lighted chamber. Two ANOVA tests were used to compare the first 4 trials (p>0.05) and the first five trials (p<0.05). The 1st and 5th trials were compared using the t-test (p<0.05). Error bar represents s.e.m. b) Avoidance time vs. sex (n=6, 4 females and 2 males). Avoidance time is the time that both male and female flies stayed in the dark chamber. This figure shows average avoidance time by both male and female flies to enter the lighted chamber. Males and females were compared using a t-test (p>0.05). Error bar represents s.e.m. c) Average trials to learn in fish oil and standard diet (n=18, Group A=9, Group B=9). Learning trial is considered as the first of any two sequential trials that fly takes longer than 30 seconds. A t-test was used to compare between fish oil and standard diet (p<0.05). Error bar represents s.e.m.
Discussion
The purpose of this study was to determine whether fish oil had an effect on the learning and memory in D. melanogaster. Based on the results, the time taken by the positively phototactic flies to enter the lighted chamber increases with the number of trials, indicating that they are learning (Figure 2a). The results indicating that the flies had started learning by the 5th trial (Figure 2a) were similar to a study that claimed that wild type flies avoid the lighted chamber after 3 to 5 training trials (Ali et al., 2011). As the fly enters the lighted chamber, it comes into contact with the quinine hydrochloride solution which creates an aversive stimulus. In the initial trials, the flies moved to the lighted chamber within a few seconds, but as the number of trials increased, the flies learned to avoid the lighted chamber as indicated by the increase in the time taken to enter the lighted chamber (Figure 2a). In the pilot study, the flies were separated based on their sex difference. The results showed that there was no significant difference between the male and female flies in the time taken to learn (Figure 2b). Therefore, for the study (Figure 2c), the flies were not separated based on the sex difference. The results of the test show that the flies exposed to fish oil learned faster than the flies grown in the standard diet (Figure 2c). The flies in the fish oil group took fewer trials to learn when compared to the flies of the standard diet group. These results are in accordance with a study done on rats which showed that fish oil supplementation during brain development and adulthood in normal rats enhanced their memory (Chung et al., 2008).
Fish oil contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Human beings need EPA and DHA to develop and function normally (Swanson et al., 2012). In human beings, EPA and DHA are produced using alpha-linolenic acid (ALA) which is an essential fatty acid obtained from the diet. Only a small amount of DHA is produced from EPA, which is produced from ALA (Goyens et al.,2006). Therefore, it is necessary to obtain DHA through diet. It is known for its role and support in brain function (Swanson et al., 2012). In our experiment, the positive effect of fish oil on the learning of D. melanogaster could have been due to the presence of EPA and DHA fatty acids in the fish oil. Studies show that EPA and DHA have been linked to promising results in the cognitive function in people with very mild Alzheimer’s disease (Swanson et al., 2012). A study showed that due to the lack of D-6 and D-5 desaturases, the key enzymes for EPA and DHA biosynthesis, D. melanogaster cannot biosynthesize EPA and DHA (Shen et al., 2010). Introducing these fatty acids in D. melanogaster through fish oil might have helped strengthen the synapses between neurons, which could have resulted in faster learning.
The flies grown in fish oil were active; however, some of the flies in the vials with fish oil diet died. One fly reared in fish oil and used for the experiment died a few hours after the experiment. Another interesting observation during the study was that even two weeks after introducing male and female adults in vials containing fish oil, no larvae were observed. The initial plan was to use the flies grown from the larvae produced by the flies in the fish oil vial; however, no larvae were obtained. The larvae from the standard diet vial were then transferred into the fish oil diet vial, but even after transferring them, no flies were observed. The larvae were exposed to high levels of fat in the food which may not have been optimal for larval growth. Because of these challenges, the young flies were transferred from the standard diet vial into the fish oil diet vial and were exposed to the fish oil for at least a week. The flies were then used for the experiment.
There were some limitations to this study. The filter paper that was used as an aversive stimulus did not cover the entire lighted chamber. In order to see the fly entering the lighted chamber, part of the lighted chamber was not covered with filter paper intentionally. For the future experiments, we can overcome this problem by using a transparent filter paper. A small amount of light dissipated from the light chamber into the central chamber. Some flies stayed in the central chamber of the t-maze where light was present, but quinine was not. Since the light in the central chamber was not very bright and because there was no quinine, it was considered to be the dark chamber. By the time the training was started, the starvation time for all the flies was not the same. All of these limitations might have had an effect on the overall results. During the first few training trials, the flies in the fish oil diet moved into the lighted chamber within a few seconds, but during the later trials, they seemed to avoid the lighted chamber. This might have happened because of the taste or smell of fish oil present in the fish oil diet vial. The flies might have disliked the smell or taste of fish oil, which could have acted as aversive stimulus. If this was the case, then since they were already exposed to the aversive stimulus (fish oil), this might have increased their chances of avoiding the quinine solution used as an aversive stimulus in our study. This still supports our hypothesis that fish oil improved learning because the flies entered the lighted chamber quickly during the initial trails and they only started avoiding the lighted chamber after the first few trails. Exposure to the quinine solution during the initial trails is enough for them to learn and associate it with light. For the future, we would suggest using an electrical shock or scent as an aversive stimulus instead of using quinine solution.
Acknowledgements
The authors would like to thank Drs. Kwangwon Lee and Nathan Fried for teaching the PPBR course and their support, comments and suggestions throughout the project. We would also like to thank Sarah Johnson for ordering quinine hydrochloride. Finally, we would like to thank Rutgers University Camden for providing us with lab space and supplies.
References
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
