Heather Stang and Oksana Tkachuk
Department of Biology, Rutgers University, Camden NJ 08102
Abstract
It has been of recent interest to investigate agricultural methods that will not only benefit plant health, but will ultimately not cause harm to them as well. Previous methods such as pesticides and fertilizers have been popular in growing healthy plants; however, they have negative effects on the soil. One alternative to the use of chemicals is compost soil, a planting material with beneficial microbes that aid in the production of strong and healthy plants. The microbial communities present helps plants retain water and hold on to nutrients. These microbes are crucial for growing healthy plants and without them, the plants would subsequently die. This study looks at the importance of the microbial community in soil for plant health, specifically in the model organism Lathyrus odoratus. Three different soil types were used and compared in their natural form and sterilized form to determine the effects of the microbial communities. Based on our results, we concluded that the microbial community in soil play a major role in overall plant health and can be an alternative to the use of chemicals.
Introduction
Microbial communities are crucial in agricultural growth for plants to be able to acquire nutrients, have immunity from potential pathogens, as well as provide optimal growth (Bainard 2013). These biotic factors are required in soil and interact with the abiotic factors to allow the plants to grow (Pérez-Piqueres 2006). A condition absent of microbes would cause all plants in the soil to die due to the inability to retain nutrients. Microbial communities can be leeched from the soil in several ways. One common way is during severe weather, where soil erosion takes place and pulls the plant out of the earth (Deng 2010). Humans can cause damage as well from slash and burn techniques that kill microbes that have grown over time from surrounding plant species (Deng 2010). Farmers try to control their crops to prevent soil degradation from happening in order to promote the growth of healthy plants.
Depending on geographical location, different soil types permit optimal growth for certain plant species. Some plants require loose sandy soil, where some require dense, moist soil (Pérez-Piqueres 2006). Regardless of the crop, to compete with its demand, many farmers use chemicals to enhance plant growth. While chemicals and pesticides may produce faster growing plants, it slowly degrades the microbial community that naturally forms in the soil (Bainard 2013). Due to popularity in chemical use, little has been studied comparing microbial communities in local, natural soils that have not been treated with pollution or chemicals.
If traditional forest soil from the Mid-Atlantic region, sandy soil from the Pine Barrens, and certified organic compost soil are taken and compared, how would their microbial communities differ? Compost soil is known to improve growing conditions with its high microbial count and yield healthier plants (Pérez-Piqueres 2006). However, if tested against the mid-Atlantic forest soil and sandy Pinelands soil, would the enriched soil in fact grow healthier plants? This study focuses on answering this question by directly comparing the effects of plant fitness with the three types of soil mentioned. The experiment uses the model organism L. odoratus, as it is a popular crop that is easy to grow. We hypothesize that a higher microbial population will yield stronger, healthier plants, aiding in water and nutrient retention, as well as immunity from potential pathogens.
Materials & Methods
Plant Material, Different Soils, and Growth Conditions
Twenty one sprouts of L. odoratus were obtained, along with the three types of soil for potting: organic compost soil, purchased from gardening store; natural dirt, taken from uncontaminated location; sandy soil, taken from pinelands. Portions of each type of soil collected were dry autoclaved for 20 minutes cycles. The plants were extracted from the original soil and washed to remove any residual dirt prior to potting. Three plants were placed in each type of soil. The total number of pots used was six; three autoclaved samples in each soil type and three non-autoclaved in each soil type. Plants were placed in the greenhouse and were kept under natural light conditions, constant temperature, and were watered daily using reverse osmosis filtered water with added minerals.
Determining the Health of L. odoratus
To correlate the effects of microbial communities on the health of L. odoratus, a couple different assays were performed. First, a qualitative assay was carried out by taking pictures of each plant and comparing their appearances visually. Second, a quantitative assay was completed by measuring the leaf surface area and number of plant branches over 14 days. On the 14th day, the sprouts were extracted and washed to remove any soil. Each sprout was subsequently massed as the final determinant to assess growth.
Culturing and Estimating the Microbial Count in Soil
To determine if microbial communities were present in the samples, a direct plate count assay was performed on each soil type. This method comprised of collecting 5 g of soil from each pot and diluting it with 40 ml of sterile water. Each sample was inverted 10 times and left for 10 minutes to settle. The samples were then diluted with sterile water in the following proportions: 2 μL sol to 98 μL water and 5 μL sol to 95 μl water. Two dilutions were created in case one type failed or produced too high of a colony. A spread for every soil type was prepared in both dilutions on Potato Dextrose Agar plates, creating 12 total plates. The plates were set out for five days in a lab at room temperature. On the fifth day, the plates were visually observed for a direct count.
Results
To test if the presence of a microbial community affected plant fitness, different natural soil samples were compared to the corresponding autoclaved samples. After the sprouts were potted, it was observed that the plant conditions started to transform on the second day of the experiment, as seen in Figure 1A. The number of branches in the natural samples increased and these plants had an overall healthy appearance. On the other hand, it was evident that the plants in the autoclaved samples were deteriorating. The leaf measurements were taken daily to assess the plant growth rate, but it did not show any significant difference. This study attempted to assess the microbial community numerically in each soil sample by the direct count method, but the results were inconclusive. At the end of the study, the mass of the plants were taken and placed into a graph for comparison, seen in Figure 1B. There was a significant difference between the masses of the sprouts grown in the natural soil samples compared to the autoclaved samples. A 2-tailed T-test was performed for the three soil types, and all three yielded a p value less than 0.05, indicating that there is a correlation between soil microbes and plant health.
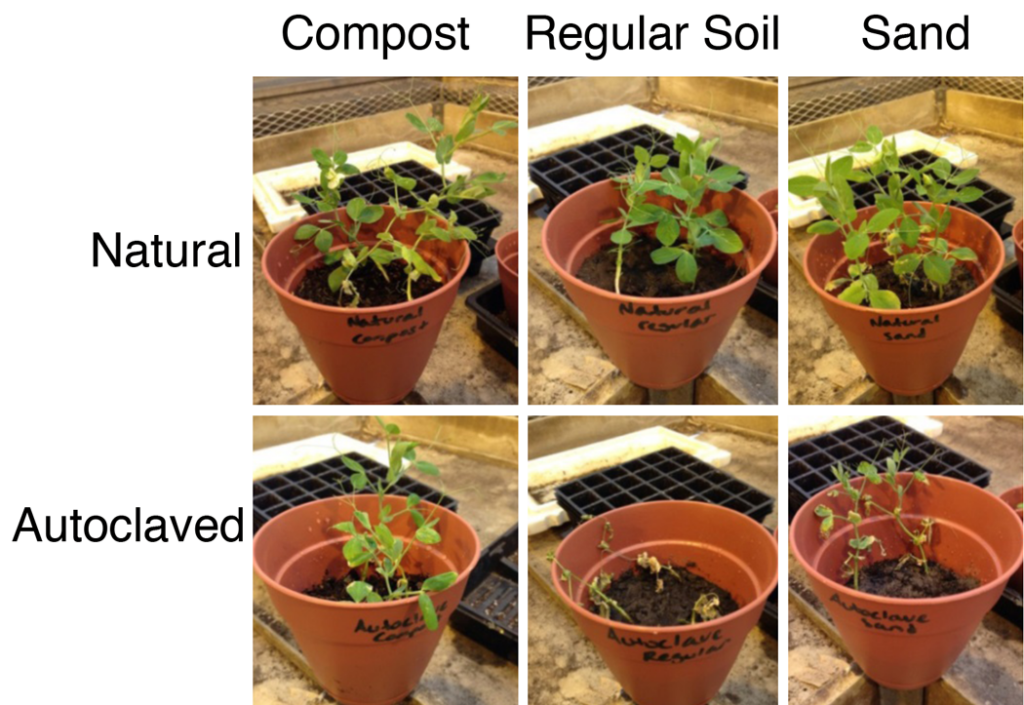
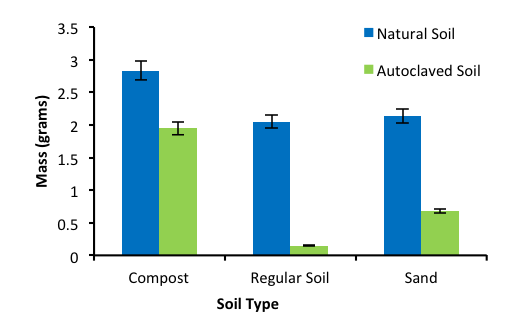
Figure 1. Average plant mass in different soils. A. Images of plants in different soils. B. Quantitative measurements of plant mass. Each soil type studied comparing natural to the autoclaved samples on the 14th day of the study. Error bars represents one standard deviation. Plants in natural soils produced more cell mass that those in autoclaved soils in all soil types (two-tailed t-test, p
Discussion
Based on the qualitative data seen in Figure 1 and 2-tailed T-test taken for each soil type, there appears to be a direct correlation between plant health and the microbial community present in the soil. The natural soils contain plants that are healthier in terms of size and appearance compared to their sterilized counterparts, which were deteriorating at a rapid rate. These results support the hypothesis that microbial communities are beneficial for growing healthy plants. Therefore, methods that focus on increasing the microbial communities already present in soils can be implemented in agricultural techniques in lieu of the use of chemicals. To further confirm these results, testing different plant species and using different soil samples over longer periods of time should be performed.
The method of measuring leaf size to quantifiably show plant growth or deterioration was ineffective. This can be attributed to the fact that leaf size did not necessarily shrink as certain plants began to deteriorate. Measuring individual plant mass proved to be an effective technique to show resulting plant health after being tested in the various soils. For any future studies, mapping plant surface area throughout the life cycle through technological monitoring could be a more effective method of directly observing changes in plant health.
Attempting to observe microbial communities present in the soil types through direct count plating was also determined to be ineffective. Microbes did grow on the plates, however, some plates, especially from the natural soil samples, produced long colonies that made it visually impossible to make a direct count. This accounted for the results being inconclusive, as no quantitative measures of microbial populations could be taken. If repeated, the plates should be placed in a colder environment to hinder microbial growth. The colonies should also be counted within a shorter period of time to ensure a smaller number of colonies that could be counted.
For the future direction of the experiment, microbes should be added to the autoclaved samples after a period of time to look for signs of regeneration. This could further show the advantages of microbial communities. Due to an insufficient time period, we were unable to attempt this additional stage of the experiment.
Acknowledgements
This study was performed as part of the requirement for the General Microbiology Laboratory course at Rutgers University – Camden. We would like to thank Dr. Kwangwon Lee for providing provisions for the attempts at our direct plate count, as well as his tips and advice throughout the experiment. We would also like to thank Dr. Simeon Kotchoni for providing us with an excellent greenhouse environment to grow our plants.
References
· Bainard, L. D., A. M. Koch, et al. (2013). “Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems.” Plant and Soil 363(1-2): 345-356.
· Deng, H., B. Zhang, et al. (2010). “Long-term effect of re-vegetation on the microbial community of a severely eroded soil in sub-tropical China.” Plant and Soil 328(1-2): 447-458.
· Pérez-Piqueres, A., V. Edel-Hermann, et al. (2006). “Response of soil microbial communities to compost amendments.” Soil Biology and Biochemistry 38(3): 460-470.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
