Michael Dumont, Sung Kim, Merve Ozbas, Mario Rivera B. Department of Biology, Rutgers University, Camden, NJ 08102 Edited by Daniel Pinolini
Abstract
Bt maize, a genetically modified variety of corn, produces a natural toxin made by Bacillus thuringiensis (Bt). This species of bacteria has been engineered to target the digestive lining of a small variety of insects such as worms and caterpillars, for pest control. Although there is strong insect specificity in Bt maize, it is important to verify the potential effects this toxin could have on non-target organisms and consequently, ecological systems. Crickets play key roles in the environment as weed, seed, and decayed matter consumers, as well as represent important food resources for a large variety of organisms such as birds and other large insects. This study investigated how the ingestion of Bt toxin in corn leaves could affect cricket mortality and feeding behavior. Crickets were fed diets with varying amounts of Bt corn leaves. Daily mortality rates and feeding behavior were recorded over the course of 7 days. Results of this study showed a significant increase in cricket mortalities between control and 90% Bt concentration diets (P < 0.007) and between 25% and 90% Bt concentration (P < 0.007). Results regarding feeding behavior were not entirely conclusive, as these observations may have been due to lack of adequate nutrition in corn leaves rather than a direct effect of Bt poisoning. Feeding behavior and timelines of deaths suggest Bt toxicity may have been responsible for mortalities. If crickets are susceptible to Bt poisoning, it could have significant adverse effects on wildlife that depend on cricket ecosystem interaction.
Introduction
For the past 20 years, biotechnology has produced many varieties of genetically altered crops. Genetically modified maize, commonly known as corn, is grown in approximately 92% of all planted corn crop acreage (Clive, 2015). Varieties of GMO corn include Bt corn, which has been injected with insecticide-producing bacterial DNA. The Bt variety of genetically modified corn is aptly named because of its ability to naturally produce insecticidal toxins. The label “Bt” refers to the bacteria Bacillus thuringiensis. This bacterium produces a toxin, such as CRY1AB, which is used as a natural pesticide against a small variety of insects, including mosquitos, moths and bore worms (Ibrahim et al., 2010). Bt genes are inserted into the corn genetic material and cause the corn cells to naturally produce pesticides, which are then expressed in every cell of the plant. This pesticide attaches to protein receptors in cell membranes of the insect’s gut and dissolve them, effectively paralyzing the digestive system and starve the insect pests. Despite this toxin supposedly affecting only certain insects considered pests, studying the effects of these genetically altered crops on a wide variety of insects is important to properly evaluate their impact on wildlife and ecosystems. Some studies suggest that Bt toxin may not be as target specific as initially thought. In one study, ingestion of Bt toxin increased the mortality rate of ladybug Adalia bipunctata (Schmidt et al., 2009). Another study reported adverse effects on the growth of caddisflies (trichopterans) due to their close relation to targeted butterflies and moths, (lepidopterans) (Rosi-Marshal et al., 2007). One insect that may be adversely affected by Bt toxin is the cricket (Gryllidae). Crickets are omnivorous insects that can be commonly found in crop fields, pastures, weedy areas, roadsides, and lawns (Carmona et al., 1999). They primarily consume living and dead matter, which may include other insects, plants, grass, and seeds. Some studies suggest that crickets may function as pests, feeding on cabbage, green peas, and soft corn grains (Criddle, 1925). However, several lines of evidence have shown that crickets are responsible for removing a significant portion of ragweed and redroot pigweed seeds from crop fields while ignoring wheat seeds, demonstrating their potential use as a natural weed-killer (Carmona et al., 1999, Brust et al., 1988). Other studies have shown that crickets act as natural pest deterrents, feeding on grasshopper eggs, beetle eggs, and ootheca of praying mantids (Monteith, 1971). In apple orchards experiments, crickets were found to extensively hunt and feed on apple maggots (R. pomonella pupae) as well as damaged fruit whilst ignoring the fresh apples (Monteith, 1971). Oftentimes, the crickets would only briefly feed on fallen damaged apples, then move off in search of prey under the soil surface. Overall, crickets can either harm or help crop health depending on what food items are available. Due to their widespread prevalence, pest predation, role in weed and decaying plant matter removal, and their importance in the food chain as a prey item for birds, reptiles, and other large insects, it is important to study how Bt toxin may affect crickets. Due to their close familial relation with pests such as grasshoppers, we predict that cricket populations exposed to a Bt-positive diet will have a higher mortality rate than crickets fed a non-Bt diet.
Materials & Methods
Corn Treatments and cricket mortality and feeding rate experiments Bt-positive Corn leaves were sourced from Little Hooves Romney Farm in Moorestown, NJ. These leaves were baked at 60˚C for 24 hours to dry prior the feeding experiments. The dried leaves were very finely chopped to be mixed with Fluker’s High-Calcium Cricket Diet powdered food, as well as to assimilate to its consistency. To test the effects of Bt on insects not considered pests, we used house crickets (Acheta domestica) as a study model. House crickets were supplied from local Petco suppliers on the first day of experimentation. Crickets were housed in four separate, large lidded Tupperware containers, which were kept at room temperature. Petri dishes with 3 grams of food material made of corn leaves and two empty cardboard paper tubes (approximately 10.5cm long) were held in the containers to provide the crickets with hiding spots. Drinking water was provided in a petri dish with cotton balls soaked in bottled water, to avoid crickets drowning. Each container received 3g of a specific food treatment; control groups were exclusively given Fluker’s High-Calcium Cricket Diet powdered food, while treatment groups were fed with ratios of 25%, 45%, or 90% Bt-positive corn leaves mixed with cricket food. Each trial consisted of a control group and 3 experimental groups. Each group housed 2 small, 2 medium, and 2 large crickets per Tupperware container. Crickets were fed for 7 consecutive days, and three trials of the experiment were conducted. Water availability was checked and replaced daily as needed. Food consumption was measured at the conclusion of the experiment by subtracting the final remaining food mass from the 3 grams initially provided. Cricket mortalities were recorded daily and dead crickets found in the containers were removed immediately, as the carcasses could be toxic to healthy crickets.
Results
Mortality rates and time-mortality assays Mortality rates increased as concentrations of Bt increased. Average mortality rates were highest at 94% in the 90% Bt concentration group when compared to control (P < 0.007) and to 25% treatment (P < 0.007). Lowest mortalities occurred at 22% in both control and 25% treatment group. Average mortalities in 45% Bt concentration group increased slightly to 33% when compared to control and 25% Bt concentration groups (Fig. 1). Most cricket mortality occurred near the end of each trial (Fig. 2). ANOVA analysis of mortality across treatment groups reported df=3, F=10.38, and P <0.004. Tukey’s Post-Hoc analysis showed significant differences between control and 90% Bt concentration (P < 0.007) and between 25% and 90% Bt concentration (P < 0.007). Control through 45% Bt concentration treatments showed no significant differences. Overall, there appeared to be no major effects of Bt concentration on cricket mortality until treatment was at 90% concentration when compared to control and 25% treatment. Food Consumption As concentrations of Bt in plant matter increased, the crickets consumed more food (Fig. 3). Control groups consumed an average of 15% of the 3 grams of initial food provided, while 90% Bt concentration groups consumed an average of 69% of the food (P<3.92xe-05). The 25% Bt concentration group consumed an average of 27% of their food, almost double the control, and the 45% Bt concentration group consumed 56% of their food. ANOVA analysis of food consumption across treatment groups reported df=3, F=43.14, and P=2.76e-05. Tukey Post-Hoc analysis reported significance differences between 25%-45% group (P<0.003), 25%-90% group (P<0.0003), and control-45% (P<0.0003).

Figure 1. Cricket mortalities increasing as concentrations of Bt corn material in diet increased. Error bars represent standard deviation within treatment groups.
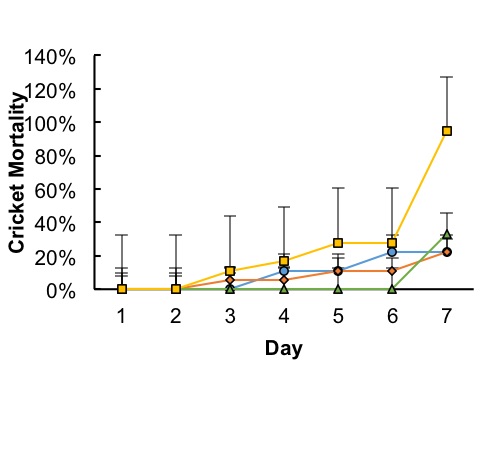
Figure 2. Timeline of mortalities by day of observation. Most deaths occurred after day 4, with an acute incidence of death at day 7 in 90% Bt treatment groups (Square) and in 45% Bt treatment groups (Triangle). Mortalities in Control groups (Circle) and 25% Bt treatment groups (Diamond) were statistically insignificant. Points represents each treatments average mortality rate with error bars representing standard deviation within treatment groups.
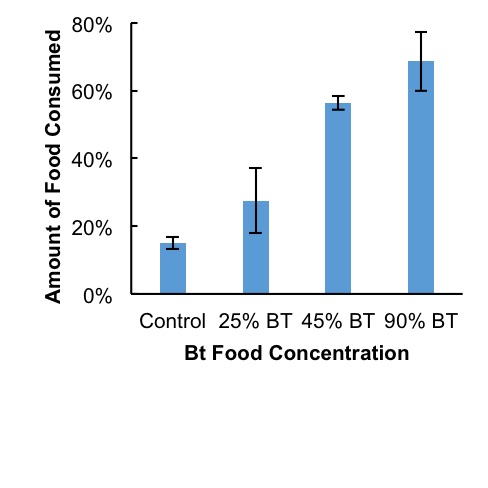
Figure 3. Larger amounts of food were consumed as diets increased in amounts of Bt corn material. Error bars represent standard deviation within treatment groups.
Discussion
In this study, we assessed the effect of Bt corn on cricket toxicity and feeding behavior. Our results showed that consumption of Bt toxin had a negative effect on the survival of the house cricket. The effect of Bt corn was evidenced by increased mortality rate of crickets in 45% and 90% concentration of Bt in their diet (Figs. 1 and 2), as well as in the crickets feeding rates (Fig. 3). The increased mortality of crickets consuming Bt corn suggests that this toxin is less specific than previously thought. Early deaths among control and low dosage groups remain unexplained. However, these deaths remained somewhat consistent and were likely due to natural causes. Assertions that Bt caused treatment mortalities act contrary to the generalized assumption that Bt toxin is targeted towards specific pests (O’Callaghan et al., 2005). Crickets consume pests, weeds, seeds, and decaying matter, rarely functioning as influential pests and should not be affected by Bt toxicity. Our results suggest that Bt may have a larger spectrum of activity than previously thought, affecting insects beyond crop pests. There appeared to be a positive correlation between total food consumed and Bt concentration (Fig. 3). The increase in food consumption may suggest that crickets were not meeting their nutritional needs to survive and began increasing their food intake to compensate. Overfeeding due to lack of adequate nutrition could be due to the Bt toxin destroying their stomach lining, causing an inability to digest their food (Ibrahim et al., 2010). However, the increase in consumption may have also been due to crickets preferring the taste of plant material over Fluker’s High-Calcium Cricket Diet powdered food. Death due to lack of nutritional fulfillment was further evidenced in the timeline of mortalities (Fig. 2). Most deaths in 90% concentration groups occurred at the 7-day mark in all trials, suggesting a delayed but very notable response to treatment. As stated above, these observations may have been because of Bt toxicity or a lack of nutrition in corn leaves. Future research should aim to better correlate Bt poisoning with cause of death among crickets in similar lab settings. Due to a poor selection of control measures, results from this experiment were not conclusive in attributing mortalities to Bt toxicity. If this experiment should be repeated, treatments should be organized such that diets among controls and treatments remain the same except for the presence or absence of Bt. Death due to disruption in digestive action by Bt could be further measured by taking mass differences of crickets, or by examining their stomach lining under microscope for evidence of stomach lining ruptures and Bt crystallization in the cell membranes. Further quantitative field research regarding how much Bt toxin is in consumed plant material, as well as the likelihood that crickets will consume a lethal dose would be beneficial. While the crickets in this study did seem to prefer samples containing more toxin, this may have been due to a lack of palatability in commercial powdered food combined with a lack of alternative food sources. Comparisons between food preference of Bt products and other food sources, such as seeds, weeds, crops, decaying matter, and live prey may add weight to the assertion that the widespread prevalence of Bt corn is a threat to cricket populations.
Acknowledgement
We would like to thank Dr. Kwangwon Lee and Daniel Pinolini for their continued support in the achievement of this project. Special thanks to Dr. Angélica L. González for her constant guidance and assistance and to Dr. Amy Savage for providing us with invaluable advice as well as allowing us to use her lab space for the duration of our experiment. Lastly, we would like to thank the respective lab members from each of the labs mentioned above. Their insight and assistance in this research endeavor was valued and greatly appreciated.
Reference
Brust, G.E., and House, G.J. (1988). Weed seed destruction by arthropods and rodents in low-input soybean agroecosystems. Am. J. Altern. Agric. 3, 19.
Carmona, D.M., Menalled, F.D., and Landis, D.A. (1999). Gryllus pennsylvanicus (Orthoptera: Gryllidae): Laboratory Weed Seed Predation and Within Field Activity-Density. J. Econ. Entomol. 92, 825–829.
James, Clive. 2015. 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015. ISAAA Brief No. 51. ISAAA: Ithaca, NY.
Criddle, N. (1925). FIELD CRICKETS IN MANITOBA. Can. Entomol. 57, 79–84.
Ibrahim, M.A., Griko, N., Junker, M., and Bulla, L.A. (2010). Bacillus thuringiensis. Bioeng. Bugs 1, 31–50.
Maureen O’Callaghan, Travis R. Glare, Elisabeth P.J. Burgess, and Malone, L.A. (2005). Effects of Plants Genetically Modified for Insect Resistance on Nontarget Organisms. Annual Review of Entomology 50, 271–292.
Monteith, L.G. (1971). Crickets as predators of the apple maggot, Rhagoletis pomonella (Diptera: Tephritidae). Can. Entomol. 103, 52–58.
Rosi-Marshall, E., Tank, J.L., Royer, T., Whiles, M.R., Evans-White, M.A., Chambers, C.P., Griffiths, N.A., Pokelsek, J., and Stephen, M.L. (2007). Toxins in transgenic crop byproducts may affect headwater stream ecosystems. Proceedings of the National Academy of Sciences 104, 16204–16208.
Schmidt, J.E.U., Braun, C.U., Whitehouse, L.P., and Hilbeck, A. (2009). Effects of Activated Bt Transgene Products (Cry1Ab, Cry3Bb) on Immature Stages of the Ladybird Adalia bipunctata in Laboratory Ecotoxicity Testing. Arch. Environ. Contam. Toxicol. 56, 221–228.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
