Jenny Pan, Taylor O’Rourke, Meghan Wachira, Manpreet Kaur, Ubaidah Khan
Department of Biology, Rutgers University, Camden, NJ. 08102
Abstract
Pain is described as an unpleasant sensory and emotional experience correlated with tissue damage. Pain can manifest itself into several forms such as acute and chronic. While aging is often accompanied with pain, little is known about how the aging process impacts each. Currently, there are conflicting studies on how pain sensitivity changes over a lifetime. Various studies suggest that pain perception decreases as an individual ages, while opposing studies suggest that pain perception increases with age. Our study uses thermally induced noxious stimuli to test pain among various age-groups in Drosophila melanogaster. The short life span of D. melanogaster allows for age-related research to be conducted. Through the use of an acute thermal pain-assay, we hypothesize that aging will decrease pain perception in D. melanogaster but will increase in chronic pain. Our first aim is to determine how age plays a role in acute thermal sensitivity by exposing D. melanogaster to a noxious temperature of 42°C. The second aim is to determine how age affects thermal sensitivity in chronic pain through the amputation of the right mesothoracic leg of the fly. This proposal will aid in developing an animal model to further explore the mechanisms behind how age affects pain perception. Ultimately, the wide-ranging impact of this study could lead to the development of novel pharmaceutical interventions for pain patients that takes age into account.
Specific Aims
Pain is a distinct and reliable signal to our body indicating that harm or damage has occurred. It can be subdivided into chronic and acute pain. Chronic pain poses an enormous national healthcare problem, costing the nation nearly $600 billion and affecting approximately 100 million individuals (Gaskin and Richard, 2012). Acute pain is directly related to injury or tissue damage and eventually disappears. Chronic pain, however, is initially caused by an injury or damage but continues long after the injury heals. There is currently no known cure for chronic pain.
Existing studies show that as individuals age, particularly those over 65, the risk of suffering from chronic pain increases significantly (Millecamps et al., 2019). Currently, the elderly are defined as individuals 65 and older, yet the definition seems to be changing to qualify only individuals 75 and older as the elderly (Ouchi et al., 2017). For the purposes of this paper, the elderly are defined as individuals 65 and older. It is projected by the United States Census that by 2030, nearly 20 percent of the US population will represent the elderly. As the elderly population grows, the percentage of chronic pain patients in this population will increase. However, a significant number of individuals that experience chronic pain may go unidentified by medical professionals. Chronic pain in elderly individuals is often not detected, leading to a lack of treatment of chronic pain states in these individuals (Kaye et al., 2010). In a geriatric nursing home, researchers found that 66% of the residents dealt with chronic pain, yet 34% of the cases were undetected by physicians (Kaye et al., 2010). This presents compelling evidence that more research on the detection and management of chronic pain in elderly individuals needs to be conducted. However, prior studies have struggled to evaluate if there are relevant differences in the detection of chronic and acute pain states as a function of age, as a means to ameliorate current rates of detection of chronic pain in elderly individuals.
We propose the use of D. melanogaster as an animal model to utilize in these experiments. The use of fruit flies in laboratory research has proven to be very practical. The ease with which fruit flies reproduce and speed in which they age make them a suitable model organism to studying the effects of aging. Additionally, a drosophila thermal sensitivity assay has been developed and validated for assessing pain (Neely et al., 2011). Thus, it is possible to utilize drosophila to study the effects of age on pain. Further, D. melanogaster pain genetics have been extensively studied with a knock-out gene identified, painless, that can be utilized as a negative control in pain-related studies (Im and Galko, 2012).
Previous studies demonstrated tissue damage induces thermal sensitivity in Drosophila melanogaster (Babcock et al., 2009). The conservation of human disease genes in Drosophila melanogaster presents a valuable opportunity to perform age-related studies. D. melanogaster orthologues to human aging genes have been identified (Brandt and Vilcinskas, 2013). Overall, we are aiming to discern the effects age has on the perception of acute and chronic pain states. By attempting to further understand if age plays a role in the experience of acute or chronic pain, we can add to discussions surrounding the treatment and detection of such states.
Aim 1: Determine how age affects acute thermal sensitivity in Drosophila melanogaster: Drosophila will be tested using Petri dishes that are placed in a thermally controlled water bath at a noxious temperature of 42°C. Data will be collected by counting the number of flies that move away from the noxious heat during a time span of four minutes (Neely et al., 2011). Using acute pain as the primary stimulus presents a unique opportunity to identify why there could be differences in the way older and younger people experience pain. Presently, several pathophysiological changes such as the expression and functional state of spinal glia, molecular alterations in gene expression, and other biochemically related components contribute to the current understanding of the relationship between age and pain (Yezierski, 2012). This attempt to clarify consistent or distinct age-related changes in acute pain provides a reasonable foundation for understanding the effects of age on pain perception in flies. Additional research further examining the mechanisms behind the effects of age on pain can utilize these experiments as scientific backing for evidence that age may affect pain in flies.
Aim 2: Determine how age affects chronic thermal sensitivity in Drosophila melanogaster: Acute pain presents itself as a relevant topic of study surrounding the underlying differences in age-related pain. To induce chronic pain, an amputation of the right mesothoracic leg will be performed. The flies then undergo the same experimental plan as seen in Aim 1, with one crucial difference. Drosophila melanogaster are tested using Petri dishes that are placed in a thermally controlled water bath at a non-noxious temperature of 39°C in a thermal allodynic assay.
Together, these aims will characterize the impact of age on pain in D. melanogaster and will provide a useful foundation for further studying the mechanisms behind this phenomenon.
Significance
Pain is a response to a noxious stimulus, which can be affected by age. The recognition and interpretation of sensory information from a noxious stimulus is referred to as pain perception, while nociception is a neuronal response to harmful stimuli. However, pain is not always coupled with nociception since pain is a product processed by the brain. One study proposed that the ability to perceive pain decreases with age due to degeneration of brain circuits responsible for processing nociception (Ghimire and Kim, 2015). However, other studies state that aging increases pain perception due to inflammation in the immune system caused by cytokine fluctuation (Cruz-Almeida et al., 2015). Researchers observed that in the elderly, thermal-pain sensitivity is slightly diminished while pressure-pain sensitivity is heightened (Ghimire and Kim, 2015). Why one type of pain is heightened while the other is suppressed is unknown since the molecular mechanisms behind chronic pain and age are not well understood. This demonstrates that pain and its association with aging is an underexplored topic in the scientific research field. The importance of understanding the components that underpin the relation of age and pain can be applied to finding alternative treatments. Identifying the cellular and molecular mechanisms that alter pain perception in the elderly can lead to developments in age-specific therapeutic treatments.
Pain prevalence increases with age. A survey of 500 households, with individuals ranging between the ages of 18 to 105, experienced preceding pain for at least two weeks. Individuals over the age of 60 had pain lasting twice as long in comparison to those who are younger (Ferrell, 1991). Currently, there are contradictory findings on the specific effects of age on chronic pain. Using rodents ranging from 8-32 months of age, researchers studied pain sensitivity by subjecting the rats to a thermal chamber set to 10-45°C and concluded that pain sensitivity changes are due to the plasticity in the spinal nociceptive processing. These results are indicative that the threshold for pain perception increases while the tolerance decreases as age progresses (Yezierski, 2012). Other studies show varying results due to differences in pain stimuli. One study suggests that age-related immune system dysregulation occurs in response to the cytokine levels peaking later in older individuals and is responsible for why there is an increase in pain perception as we age (Cruz-Almeida et al., 2015). This study uses human experimental pain models, with the average age ranging from 21.4 to 68.1, subjected to thermal nociception. A similar study shows that the dysregulation between pro- and anti- inflammatory cytokines is the cause for the increase of pain perception as an individual ages (Malcangio et al., 2013). These studies show that pain increases in older individuals, which contradicts another study indicating an increased pain threshold in older people (Yezierski, 2012).
Pain can be split into two categories: acute and chronic. Acute pain typically lasts for a short period of time (lacerations, surgery, childbirth, dental procedures), but chronic pain lasts beyond the recovery time of an injury; its influence can span from a couple of months to years (Grichnik and Ferrante, 1991). Being able to decipher between acute and chronic pain is key in developing treatment plans. Chronic pain can be difficult to treat, as the causes of chronic pain can originate from an underlying medical condition such as diabetes. However, it can also become a disease on its own due to the nature of the pain causing a change to the nervous system over time, or other physiological changes. According to the National Institute of Neurological Disorders and Stroke (NINDS), chronic pain is linked to changes in the neuronal plasticity of the nervous system, that is responsible for an inclination of chronic pain. Psychological changes such as depression, anxiety, and mental disorders are related as consequences of chronic pain; these factors have the ability to diminish one’s quality of life (Dueñas et al., 2016). Pain, combined with these mental illnesses, can adversely affect productivity and social activities, and can lead to a decrease in one’s overall quality of life. As an individual ages, the more susceptible they are to experiencing ailments that may lead to development of chronic pain.
The CDC reported an estimate of 20.4%, approximately 50 million adults, in the United States experience chronic pain in 2016. From that 50 million, 13.6 million are elderly, ranging from the ages of 65 and above. This portion of the elderly composes 27% of all chronic pain patients (Dahlhamer, 2018). Many treatments for chronic pain vary from short-term alleviation through the usage of pharmaceutical drugs, and possible long-term alleviation with non-pharmaceutical drugs, such as exercise or acupuncture. Opioids are on a rise as a pharmaceutical drug used in pain treatment; however, they are considered high-risk drugs due to their addictive nature, along with the development of opioid-induced hyperalgesia, increased risk of falls and fractures, and respiratory depression leading to overdose death. The CDC discovered a trend which stated that in the past 15 years there was a direct correlation between the number of opioid prescriptions to the mortality rate related to opioid usage
Technological advancement has pushed towards more effective medicine. New vaccines and drugs are developed every year, with a growing success rate. These advancements have increased life expectancy, causing a growth in the elderly population. It is projected by the year 2030, that 1 in 5 people will be at the age of retirement according to the United States Census. Therefore, the amount of people suffering from chronic pain will also increase, since pain prevalence is demonstrated to increase with age. Those over the age of 60 had shown to experience pain lasting twice as long in comparison to younger individuals (Ferrell, 1991). This survey highlights the importance of finding alternative age-specific pain treatments.
Modeling how pain changes as a function of age in D. melanogaster can build a foundation that can later be used to determine the molecular and cellular mechanisms contributing to the effect of age on pain. The fruit fly has become a paradigm for biological phenomena, both physiologically and genetically. Larval fruit flies demonstrate a rolling behavior when exposed to painful stimuli, and their genomes have homologs to the human genome (Le et al., 2019). It is estimated that approximately 75% of human disease genes have conserved homologs in D. melanogaster, making them a great model organism when it comes to human disease studies (Milinkeviciute et al., 2012). Drosophila nociceptors and vertebrate nociceptors both exhibit morphological and functional resemblances with characteristics of naked-nerve endings which enables them to sense and respond to potential injury caused by tissue damage (Milinkeviciute et al., 2012). This factor, along with their powerful genetic tools and short generation time, makes D. melanogaster an important model organism in pain research. Although the larval stage is preferable when performing pain assays, the adult stage also confers advantages and are subjected to thermal stimulus assays where they display typical heat avoidance behavior (Neely et al., 2011). The majority of pain research using Drosophila melanogaster rely on the use of larvae. However, to study the impact of age on pain, it is essential to test on adult flies since the average life cycle of an adult fly is approximately 60 days while the testing period in the larval stage is only two days.
In this proposal, we will use a thermal stimulus assay to determine if there are any significant differences between older and younger D. melanogaster in relation to pain. The sensitivity of sensory systems and how their peripheral receptors diminish as age increases is a well-studied topic (Yezierski, 2012). In response to these sensory modalities, for our acute pain assay, we hypothesize that older flies will exhibit a decreased pain perception due to these peripheral receptors degrading with age causing a decrease in sensitivity. Additionally, an age-related immune system dysregulation causes higher cytokine levels in older individuals which results in an increased pain perception with age (Cruz-Almeida et al., 2015; Malcangio et al., 2013). We hypothesize that in our chronic pain assay, older flies will experience a more persistent pain from the inflicted injury. Based on the lower cytokine levels found in younger organisms, we predict the younger flies will be able to heal from the injury more effectively than older flies. This factor will result in older flies having an increased pain perception for chronic pain due to the lingering effects of the injury and the change in the immune system with age.
Approach
Our experimental plan is inspired by the schematic for high-throughput heat nociception (Neely et al., 2011). This paradigm involves the use of an adult Drosophila melanogaster thermal sensitivity assay ranging from 1-40 days old. In this assay, we will place 50 flies into a 35 mm Petri dish that is sealed with Scotch tape around the diameter to prevent water leakage and high humidity levels. The Petri dish is then placed into a water bath at a set temperature dependent on the experiment; confirmed with a thermometer. The first approach involves the use of a set noxious temperature, while the second approach requires a thermal allodynic assay, involving a non-noxious temperature. If the specimen is able to detect the noxious stimuli, they will demonstrate heat avoidance by flying towards the top of the Petri dish. If they are not able to detect noxious stimuli, they will remain at the bottom of the Petri dish and will result in an incapacitated state. This experiment is replicated with Oregon-R wild-type flies and Painless mutant flies in both acute and chronic pain assays.
Approach 1: Determine how age affects acute thermal sensitivity in Drosophila melanogaster
Acute thermal pain sensitivity is performed using Drosophila melanogaster, specifically Oregon-R wildtype flies. Due to its easily manipulated genome, comprising only 4 chromosomes, D. melanogaster is a genetic model organism for understanding molecular mechanisms of human disease (Pandey and Nichols, 2011). The nociceptors found on Drosophila melanogaster are preserved throughout metamorphosis and into adult life, making them a suitable model for assessing pain-related studies (Milinkeviciute et al., 2012). The short and simple life cycle of D. melanogaster allows for age-related research to be conducted. However, Painless mutant Drosophila melanogaster are deficient in noxious heat response. The painless mRNA encodes a protein of the transient receptor potential ion channel family. Painless is required for both thermal and mechanical nociception, the painless gene is expressed in peripheral neurons that extend multiple branched dendrites beneath the larval epidermis, similar to vertebrate pain receptors (Tracey et al., 2003). The Painless mutant fruit flies are used as a negative control for this thermal paradigm.
The thermal water bath paradigm is conducted in the dark allowing the fruit flies to adjust to the darkness for 30 minutes before the beginning of the experiment (Neely et al., 2011). As shown in Figure 1A, D. melanogaster flies of the same age group are collected and placed into a Petri dish at 35-mm in diameter that is sealed with Scotch tape. Prior to the beginning of the experiment, flies are given 30 minutes to acclimate at 20-25°C, room temperature. Different sets of flies are used per experiment to prevent the variable of previous stressors influencing the outcome. A control experiment is added to ensure that the avoidance of heat is due to an actual detection of a noxious stimuli. The control temperature is 25°C and the treatment temperature is 42°C. First, the water-bath is set to a noxious temperature of 42°C and a sealed Petri dish containing the fruit flies is floated on top of the water-bath for 4 minutes.
The flies that are able to sense noxious heat should avoid the bottom of the chamber that is touching the surface of the water bath, resulting in a positive heat avoidance response. While the flies that can’t detect the noxious temperature should remain at the bottom and be considered “incapacitated” (Neely et al., 2011). This paradigm is replicated again with a separate set of flies at the control temperature of 25°C. Both Oregon-R wild-types and Painless mutant fruit flies are used to observe thermally induced acute pain in Drosophila melanogaster.
In an acute thermal pain assay, we hypothesize that there will be a decrease in pain perception with age. The feasibility of our approach was confirmed in a pilot study (Figure 2). The pilot data shows that the younger flies have greater pain perception in comparison to older flies. This is demonstrated by a 40% difference in heat avoidance; in Figure 2A it is shown that younger flies had 60% thermal avoidance rate while in Figure 2B it is shown that older flies had only a 20% thermal avoidance rate. Based on the pilot data, we anticipate that younger flies will have a higher heat avoidance rate than older flies, as indicated in our anticipated results in Figure 3A. The control group, we expect the younger, older, and painless control groups to display a 50% avoidance rate; this is due to the temperature factor of 25°C, which shouldn’t put the flies under any environmental stress. While in our treatment groups we expect to see a higher avoidance rate in younger flies than older flies.
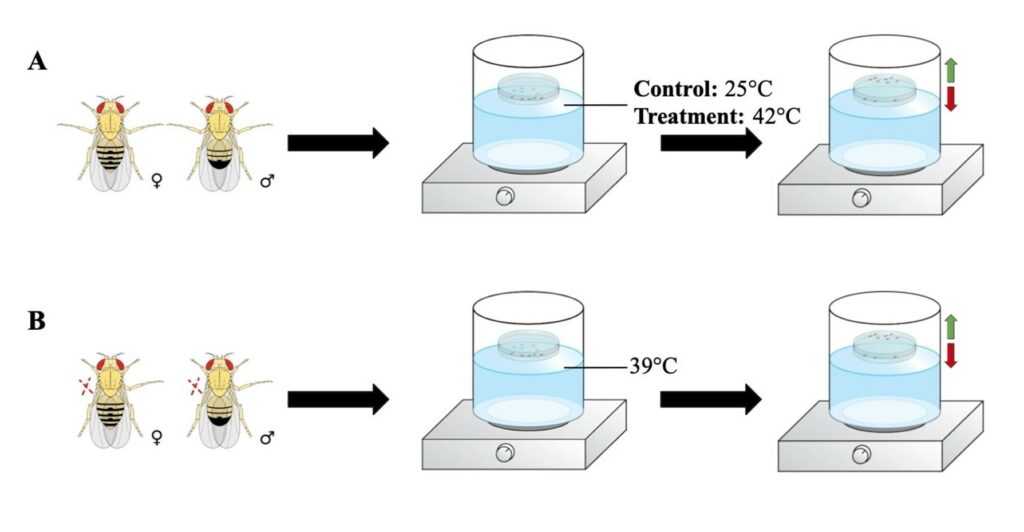
Figure 1. Thermal Nociception Assay for Acute and Chronic Pain: This diagram is a pictographic visualization of the thermal nociception assay paradigm for Aim 1 and Aim 2. The green arrows demonstrate the flies that are able to detect noxious stimuli and avoid the bottom of the Petri dish. The red arrow indicates the flies that are not able to detect noxious stimuli and in turn get incapacitated. (A) Acute thermal assay with a control temperature of 25°C and treatment temperature of 42°C. (B) Thermal allodynic assay with non-noxious temperature of 39°C.
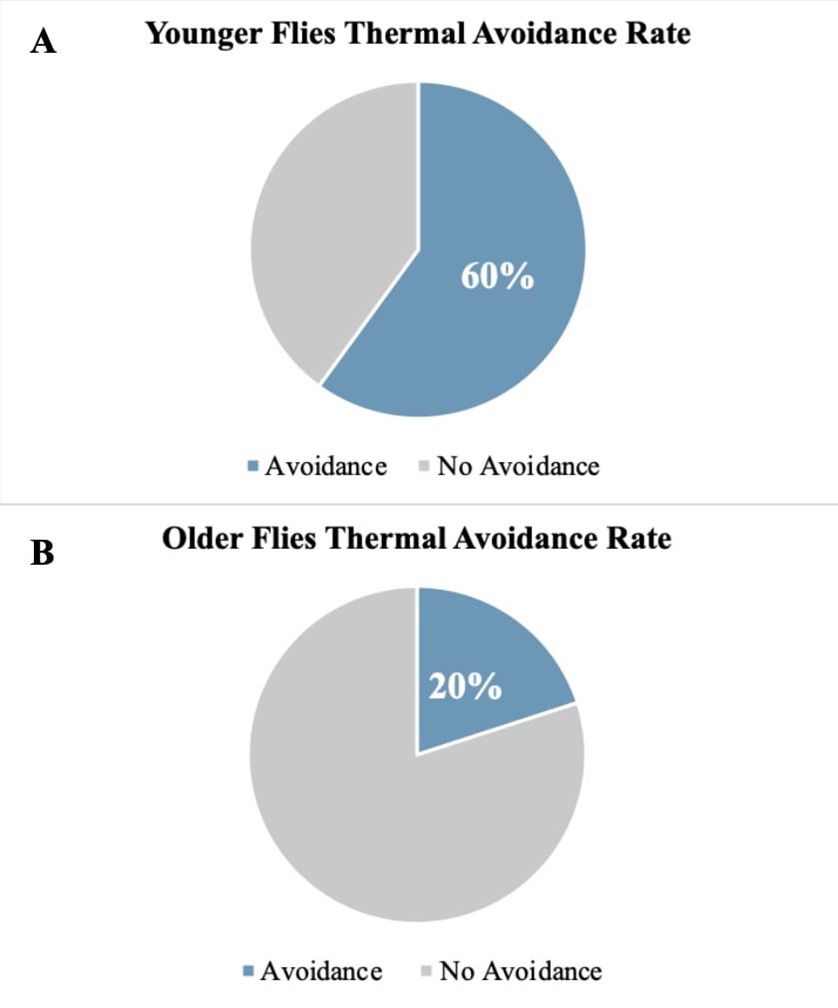
Figure 2. Pilot Data Visualizing Thermal Avoidance Rate of Acute Pain Treatment Groups: This figure visualizes the comparison of younger and older fly groups’ response to thermal water bath assay in acute pain treatment at a noxious temperature of 42°C. The blue section indicates avoidance and the grey section indicates no avoidance of noxious stimuli. (A) Shows the thermal avoidance rate of younger flies, which are less than 2 weeks old. (B) Shows the thermal avoidance rate of older flies, which are greater than 3 weeks old.
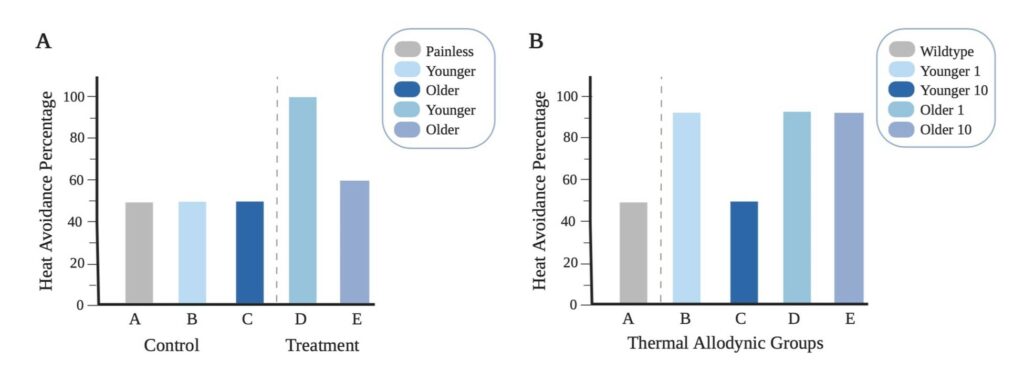
Figure 3. Anticipated Data for Acute/Chronic Pain Thermal Stimuli in Relation To Age: (A) Shows the anticipated results for acute pain assay using noxious thermal stimuli. The control is room temperature water at 25°C and treatment is thermal noxious temperature at 42°C. (B) Shows the anticipated results for thermal allodynic assay at 39°C. The control is wild-type flies without chronic pain. Young 1 and Older 1 represent the results of thermal assay the first day under the influence of chronic pain. Younger 10 and Older 10 represent the results of thermal assay ten days after the initial influence of chronic pain.
Approach 2: Determine how age affects chronic thermal allodynic sensitivity in Drosophila melanogaster
Chronic pain in D. melanogaster will be tested using thermal allodynic assay which is performed at a non-noxious temperature. This assay will detect the presence of allodynia, which is defined as a painful response to a non-painful stimulus. Implementing an allodynic experiment allows for identification of an increased sensitivity caused by the induced chronic pain. The treatment group of this experiment is split into subcategories of younger and older flies. As seen in our first approach, the younger flies will be under 2-weeks old and the older flies will be over 3-weeks old. Both treatment and control groups are subjected to a non-noxious temperature of 39°C. The control group consists of flies that do not have chronic pain thus should not experience allodynia and will not respond to the non-noxious temperature. The treatment groups are split into four categories: Younger 1, Older 1, Younger 10, and Older 10. Younger 1 and Older 1 are treatment groups that undergo the thermal allodynic assay on the same day as chronic pain infliction. Younger 10 and Older 10 are treatment groups that undergo the same assay 10-days after the initial infliction of chronic pain. We expect the younger flies to be able to recover from the injury while the older flies would continue to experience chronic pain.
Chronic pain is induced by the amputation of the right mesothoracic leg which leads to hypersensitivity. This process is demonstrated in Figure 4, where the right mesothoracic leg is amputated at the site ventral to the abdomen and dorsal to the femur using a tweezer underneath a stereo microscope at 10x magnification. The site of amputation is determined to avoid any instability in movement and mating. This removal process is completed on both the Oregon-R wild-type flies and painless mutant flies in the treatment group but is not performed on the control group. The removal procedure causes nerve injury which leads to chronic neuropathic sensitization. By damaging the peripheral nerve, a loss of central inhibition is triggered resulting in an escape of circuit plasticity and neuropathic allodynia (Khuong et al., 2019).
In a thermal allodynic assay testing chronic pain, we hypothesize there will be an increased pain perception with age. Allodynia is defined as a painful response to a non-painful stimulus. Therefore, the set temperature for both control and treatment group(s) is executed at a non-noxious temperature of 39°C as shown in Figure 1B. It is expected that the flies that are not subjected to chronic pain and do not experience allodynia, will not undergo any environmental stress from a non-noxious temperature. This will result in a heat avoidance rate of 50% due to the mere probability that the flies are either at the top or bottom of the Petri dish, represented in Figure 3B. In our treatment groups after chronic pain is inflicted, we predict the flies will initially respond with a heightened sensitivity, shown in Figure 3B. Younger 1 and Older 1 groups are expected to exhibit a similar higher heat avoidance rate on the first day due to the allodynia resulting from the chronic pain. The flies are expected to respond to the non-noxious temperature of 39°C, whereas before, as seen in our control group, the flies without allodynia will only have results due to probability. The same experiment is conducted ten days later with a different set of flies, demonstrated by Younger 10 and Older 10. The younger flies when being exposed to the same non-noxious heat 10 days later are expected to have healed from the injury and no longer experience allodynia. While the older flies are anticipated to not be able to heal from the injury and experience persistent allodynia.
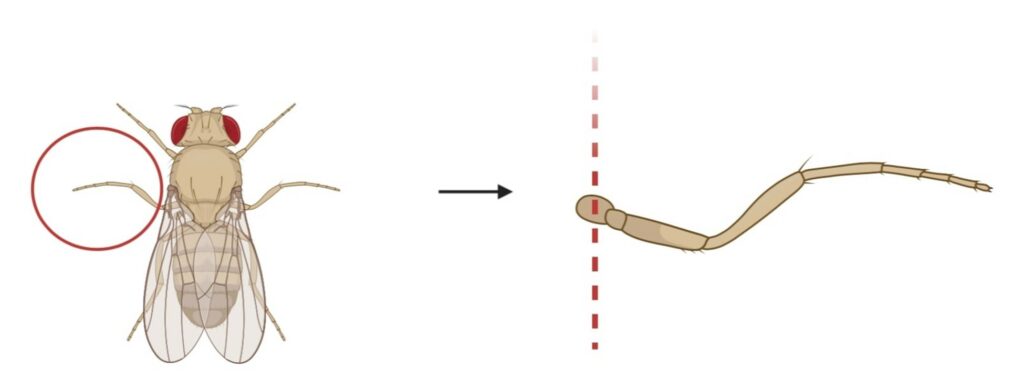
Figure 4. Amputation of Right Mesothoracic leg to Inflict Chronic Pain: This figure shows the site of amputation of the right mesothoracic leg of D. melanogaster, demonstrated with the dotted lines. The removal site is ventral to the abdomen and dorsal to the femur.
Broader Impacts
Our research proposal highlights the effects of age in relation to pain perception in Drosophila melanogaster. We hypothesize that in D. melanogaster, aging will decrease pain perception in an acute assay, but in chronic pain, there will be an increased pain perception with age. The wide-ranging impact of this study could lead to the development of novel pharmaceutical interventions for pain patients that takes age into account, particularly in the elderly population. Through the utilization of animal models, D. melanogaster can further develop into a model system that answers age and pain related questions. To find better age-specific pain treatments, a foundation must be built through animal models to prove this phenomenon exists. Our proposal serves as a basis to help create an animal model that can be used to study the mechanisms behind the effect of age on pain.
Opioids are predominantly used to relieve pain in patients; however, they contain several unique risks. These risks can include symptoms such as addiction and/or respiratory depression. Opioids are capable of slowing down breathing and eventually suppressing heart rates if used in high concentrations. The older generation may already experience discomfort due to reduced respiratory capacity and thus, they may be more susceptible to respiratory depression (Freye and Levy, 2004). Therefore, the elderly population prescribed these drugs are prone to their negative effects. We believe the basis of our research can influence future studies on the mechanism of behavioral gene expressions behind thermal pain processes. Since opioids are not the best solution to pain due to these complexities, particularly in the elderly, we hope our research proposal can establish the foundational grounds that can later be used to identify the compounds or drugs that may affect pain perception over age.
Potential future studies can expand this proposal to other pain modalities such as mechanical and chemical nociception. Researchers identified in the elderly population, thermal-pain sensitivity was marginally lower in comparison to pressure pain sensitivity, which was heightened. Given that age can affect these other modalities, we expect that changes in all forms of nociception may occur throughout the aging process. The use of different pain assays can help in understanding the neural pathways behind the different ways pain can be processed. The observation of circuitry changes that can occur in these processes may help identify the differences in sensitivity between pressure, mechanical, and thermal pain. Ultimately, in order to understand the relation behind age and pain, a foundation must be built to explore the many components that contribute to this phenomenon. Factors that range from observing changes in gene expression, to neuronal circuitry changes, and the different compounds and drugs that may affect pain perception over time are all elements that may contribute to the process of pain. These components must be determined before a significant connection can be made between age and pain. We hope that our research proposal is able to be applied as a foundational source to these potential future studies and age-specific pharmacological therapies.
Acknowledgements
We would like to extend our gratitude to Dr. Nathan Fried and Dr. Kwangwon Lee for their endless support and guidance. Even during these unprecedented times, we still received their attention and expertise with our independent research study. Without the Principles and Practices of Biological Research (PPBR) here at Rutgers Camden, we would not have had the opportunity to express our creativity and take part in a fully independent and unique undergraduate research experience.
References
Babcock, D.T., Landry, C., and Galko, M.J. (2009). Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. CB 19, 799–806.
Brandt, A., and Vilcinskas, A. (2013). The Fruit Fly Drosophila melanogaster as a Model for Aging Research. Adv. Biochem. Eng. Biotechnol. 135, 63–77.
Cole, L.J., Farrell, M.J., Gibson, S.J., and Egan, G.F. (2010). Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiology of Aging 31, 494–503.
Cruz-Almeida, Y., Aguirre, M., Sorenson, H.L., Tighe, P., Wallet, S.M., and Riley, J.L. (2015). Age differences in cytokine expression under conditions of health using experimental pain models. Experimental Gerontology 72, 150–156
Dueñas, M., Ojeda, B., Salazar, A., Mico, J.A., and Failde, I. (2016). A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 9, 457–467.
Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., Kerns, R., Von Korff, M., Porter, L., and Helmick, C. (2018). Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67, 1001–1006.
El Tumi, H., Johnson, M.I., Dantas, P.B.F., Maynard, M.J., and Tashani, O.A. (2017). Age-related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. Eur J Pain 21, 955–964.
Featherstone, D.E., Chen, K., and Broadie, K. (2009). Harvesting and Preparing Drosophila Embryos for Electrophysiological Recording and Other Procedures. JoVE 1347.
Ferrell, B.A. (1991). Pain Management in Elderly People. J. Am. Geriatr. Soc. 39, 64–73.
Freye, E., and Levy, J.V. (2004). [Use of opioids in the elderly — pharmacokinetic and pharmacodynamic considerations]. Anasthesiol Intensivmed Notfallmed Schmerzther39, 527–537.
Gaskin, D.J., and Richard, P. (2012). The Economic Costs of Pain in the United States. The Journal of Pain 13, 715–724.
Ghimire, S., and Kim, M.S. (2015). Defensive Behavior against Noxious Heat Stimuli Is Declined with Aging Due to Decreased Pain-Associated Gene Expression in Drosophila. Biomolecules & Therapeutics 23, 290–295.
Grangeteau, C., Yahou, F., Everaerts, C., Dupont, S., Farine, J.-P., Beney, L., and Ferveur, J.-F. (2018). Yeast quality in juvenile diet affects Drosophila melanogaster adult life traits. Sci. Rep. 8, 13070.
Grichnik, K.P., and Ferrante, F.M. (1991). The difference between acute and chronic pain. Mt. Sinai J. Med. 58, 217–220.
Im, S.H., and Galko, M.J. (2012). Pokes, Sunburn, and Hot Sauce: Drosophila as an Emerging Model for the Biology of Nociception. Dev. Dyn. 241, 16–26.
Khuong, T.M., Wang, Q.-P., Manion, J., Oyston, L.J., Lau, M.-T., Towler, H., Lin, Y.Q., and Neely, G.G. (2019). Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila. Sci. Adv. 5, eaaw4099.
Le, J., Shehata, M., and Slack, I. (2019). The effects of ethanol on Drosophila melanogaster mechanical nociception. The Journal of Biological Sciences 5.
Lolignier, S., Eijkelkamp, N., and Wood, J.N. (2015). Mechanical allodynia. Pflugers Arch. 467, 133–139.
Malcangio, M., Clark, A.K., and Old, E.A. (2013). Neuropathic pain and cytokines: current perspectives. JPR 803.
Milinkeviciute, G., Gentile, C., and Neely, G.G. (2012). Drosophila as a tool for studying the conserved genetics of pain: Drosophila models of nociception. Clin Genet 82, 359–366.
Millecamps, M., Shi, X.Q., Piltonen, M., Echeverry, S., Diatchenko, L., Zhang, J., and Stone, L.S. (2020). The geriatric pain experience in mice: intact cutaneous thresholds but altered responses to tonic and chronic pain. Neurobiology of Aging 89, 1–11.
Neely, G.G., Keene, A.C., Duchek, P., Chang, E.C., Wang, Q.-P., Aksoy, Y.A., Rosenzweig, M., Costigan, M., Woolf, C.J., Garrity, P.A., et al. (2011). TrpA1 Regulates Thermal Nociception in Drosophila. PLoS ONE 6, e24343.
Ouchi, Y., Rakugi, H., Arai, H., Akishita, M., Ito, H., Toba, K., and Kai, I. (2017). Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr. Gerontol. Int. 17, 1045–1047.
Pandey, U.B., and Nichols, C.D. (2011). Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 63, 411–436.
Tracey, W.D., Wilson, R.I., Laurent, G., and Benzer, S. (2003). painless, a Drosophila Gene Essential for Nociception. Cell 113, 261–273.
Yezierski, R.P. (2012). The Effects of Age on Pain Sensitivity: Preclinical Studies. Pain Med 13, S27–S36.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
