Katherine Hernandez and Haley Duncan Department of Biology, Rutgers University, Camden, NJ 08102 Edited by Joshua Waters
Abstract
To determine the potential of Strongylocentrotus purpuratus as a model for studying developmental abnormalities associated with Fetal Alcohol Syndrome, fertilized embryos were monitored through early stages of development while exposed to alcohol. Strongylocentrotus purpuratus sea urchins were spawned, eggs were fertilized, and embryos were placed into increasing concentrations of ethanol (0%, 0.02%, 0.04%, 0.06%, 0.08%, and 0.1% (v/v)) in seawater. The embryos were incubated at 13 degree Celsius and observed over a span of seven days observing embryonic developmental deformities, including the normal overall development and the formation of the gut. The embryos showed developmental abnormalities in skeletal formation, gut length, and embryonic shape as the concentration of ethanol increased. This preliminary experiment introduces the possibility of using sea urchin embryos as a model for studying the effects of alcohol consumption during embryonic development.
Introduction
Consuming alcohol during pregnancy brings a great harm to both the mother and baby. As alcohol is consumed, it enters the bloodstream and reaches the placenta where the baby resides. The fetus has a hard time breaking down the alcohol, which then begins to cause damage (Gupta et al., 2016). Fetal alcohol syndrome has been known to lead to birth defects, growth delays in height and weight, and central nervous system abnormalities. When pregnant women consume alcohol, they increase the risk of the baby developing facial abnormalities. In addition to facial abnormalities, they develop problems in their central nervous system that begin to affect the baby’s learning processes in speech, language, and attention (Gupta et al., 2016). Along with central nervous system damage, there are many other complications that come from fetal alcohol syndrome. One effect of the disorder is the disrupted/altered embryonic development of the of the gastrointestinal tract. In extremely rare cases, a link has been found between fetal alcohol syndrome and gastrointestinal neuropathy (Sujay et al., 2012). A less rare complication linked to fetal alcohol syndrome is gastrointestinal reflux disease (Sujay et al., 2012). The disease causes infants to have severe effects on their growth and nutrition that often require critical medical attention to preserve the life of the infant (Sujay et al., 2012). It can be assumed the link between fetal alcohol syndrome and gastrointestinal diseases is caused by gut abnormalities produced during embryonic development. Sea urchins are excellent subjects for developmental biology experiments because of how easy they are to spawn and fertilize (Ernst, 2011). Urchins are also useful for their transparent embryonic developmental stages and the fact sea urchins have similar genes to humans (Ernst, 2011). Recent studies have found sea urchins to be one of the top model organisms for viewing details of major tissue territories during embryonic development (McClay, 2011). Other animals that have been used for studying fetal alcohol syndrome are zebrafish, roundworms, fruit flies and frogs. Using human as subjects is not ideal because it puts the fetus at risk of irreversible complications, and abnormalities would be difficult to view during the early stages of development. Sea urchins, on the other hand, are easier to manipulate and directly view the embryos at their different stages of development (Wilson and Cudd, 2011). By exposing sea urchins to alcohol during embryonic development, we hypothesized that it will negatively impact the growth and successful development of the gut, validating urchin embryos as an alternative model for studying developmental impacts of fetal alcohol syndrome.
Materials & Methods
Live S. purpuratus specimens were Purchased from Pt. Loma Marine Lab (CA). Gametes were expressed from animals using 1.5ml of 50mM KCl. After gamete expression, it was determined whether gametes were sperm or eggs. 1ul of sperm was mixed with 20ul of eggs in filter-sterilized 1X artificial seawater (artificial seawater consists of 28.3g NaCl, 0.77g KCl, 5.41g MgCl2•6H2O, 3.42g MgSO4 or 7.13g MgSO4•7H2O, 0.2g NaHCO3, 1.56g CaCl2 dihydrate (add last) and adjust pH to 8.2, salinity should be in the range of 34-36 ppt). After a few minutes, fertilization was confirmed under an inverted microscope through visualization of fertilization envelopes surrounding fertilized eggs. 100% Ethanol was diluted to 0.02, 0.04, 0.06, 0.08, and 0.1% (v/v) in sterile 1X artificial seawater. Experimental groups of sea urchins were exposed to various percentages of alcohol after fertilization and throughout embryonic development. The control group was exposed to 1X artificial seawater with 0% alcohol throughout development in order to have a baseline growth and development schedule to compare to the experimental groups. The experimental groups were exposed to different percentages of alcohol in 1X seawater. The increments of percentages are done to possibly show different severities of complications based on the amount of alcohol to which the fetus is exposed. The percentages of alcohol were within the normal limits a woman could possibly be exposed to during a pregnancy according to her Blood Alcohol Content (0%, 0.02%, 0.04%, 0.06%, 0.08%, and 0.10%). Once placed in the proper percentage of alcohol, we observed and recorded the results of the embryonic development. We focused on final morphology rather than developmental processes. We observed any phenotypic abnormalities and any developmental effects based on the amount of alcohol to which the sea urchin is exposed. All treatments took place in covered microwell plates stored in an incubator at 13 degree Celsius. Embryos were monitored over 7 days by viewing embryos under the microscope at least once per day, to verify progression through development. On Day 7, the embryos were de-ciliated in order to accurately record the data by taking pictures of embryos in each condition under the microscope. To de-ciliate the embryos, 120 μL artificial seawater was removed from the microwells and replaced with 120 μL 2x artificial seawater, causing the embryos to shrivel up and sink to the bottom of the microwell due to the osmotic pressure. Once embryos shriveled up and sank to the bottom of the microwell, 120 μL 2x artificial seawater was removed from microwells and replaced with 120 μL artificial seawater to microwells to allow embryos to expand. Images of embryos from each treatment were captured at 40X with a microscope with a camera attached. To obtain measurements, an arbitrary scale was set using the length of a normal gut set at the same magnification as 1 arbitrary unit. Overall abnormalities were recorded and ImageJ was used to obtain measurements of the gut in each treatment. The abnormalities measured included shape, skeletal, gut and tissue abnormalities, and were represented as a percentage of embryos observed. All images were captured at the same magnification and from the same height, so an arbitrary scale could be globally applied to all images in ImageJ. Gut-length data violated assumptions of the General Linear Model (GLM), so we used the Kruskal-Wallis test to test significance, followed by multiple comparison test to look for significant differences across treatments.
Results
A variety of abnormalities were observed with increasing concentrations of ethanol (Table 1). Under normal conditions, urchins progress through development with a closed, pointed skeletal structure when viewed from the ventral side (Fig.1A), and the GI-tract develops into 3 sections, foregut, midgut, and hindgut, as viewed from the lateral side (Fig.1B). The observed abnormalities in response to ethanol included embryos with an open skeleton, as viewed from the ventral side (Fig.1C), embryos with a two-section gut, as viewed from the lateral side (Fig1.D), and embryos with tissue degeneration, as viewed from the ventral side (Fig1.E). The incidence of these abnormalities increased with alcohol exposure. Table 1 – Total Developmental Abnormalities in Response to Ethanol Treatments
| Alcohol Percentage (%) | Total Abnormalities (%) |
|---|---|
| Control (0%) | 13.2% |
| 0.02% | 21.7% |
| 0.04% | 42.9% |
| 0.06% | 57.8% |
| 0.08% | 62.1% |
| 0.10% | 75.8% |
To determine whether there is a link between gut development and fetal alcohol syndrome, measurements of the gut length ( Fig.2) were obtained . A significant difference was found among all samples, Kruskal-Wallis Test, p-value < 2.2e-16. To further characterize which treatments had significant differences among the treatments, a multiple comparison test was performed after the Kruskal-Wallis test, p = 0.05. It was found that 0.1% (v/v) ethanol was statistically significant compared to the rest of the treatments.
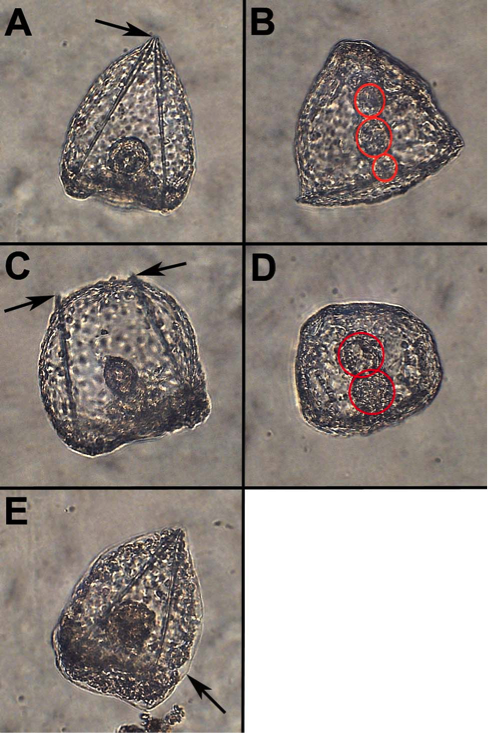
Figure 1. Developmental Abnormalities in response to Alcohol. A) Normal Embryo skeleton (Ventral view). B) Normal 3-section gut (Lateral view). C) Embryo with open skeleton (Ventral view). D) Embryo with 2-section gut (Lateral view). E) Embryo with tissue degeneration (Ventral view). Arrows point to skeleton (A & C) or degenerated tissue (E). Red circles surround gut sections.
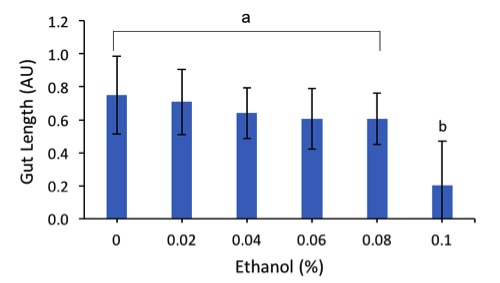
Figure 2. Average Gut Length of Embryos. This chart illustrates the average final gut lengths, in arbitrary units (AU), of embryos in response to increasing amount of ethanol (%). Error bars represent standard deviation for each ethanol treatment; letters represent treatments group by statistical significance (Materials & Methods).
Discussion
The final morphologies of the embryos appeared to support our hypothesis that increasing ethanol will interfere with normal embryonic development. Abnormalities, such as shape, skeletal, gut and tissue abnormalities were visible under the microscope in embryos exposed to ethanol. Normal shape of the embryo consists of a triangular prism with the skeleton connecting at the top of the prism, 3 visible sections of the gut, and tissues intact. The control group (0% ethanol) resulted in 13.2% of the embryo abnormalities. These abnormalities could have been due to the incubation temperature being at 13℃ instead of 15℃. Overall, it was observed that the percentage of total abnormalities increased as the percentage of alcohol exposure increased. It was also found that the average gut lengths (AU) of the embryos decreased as the ethanol (%) treatment increased. A Kruskal-Wallis test provided the p-value < 2.2e-16. Since the p-value < 0.05, the data rejects the null hypothesis and the difference was statistically significant. Therefore, our observations support that that exposure to alcohol will negatively impact the development of sea urchin embryos. Based on our results, increasing amounts of alcohol will increase the likelihood of S. purpuratus embryos being affected with abnormalities similar to Fetal Alcohol Syndrome. Our experimental treatments were based off of the Blood Alcohol Content (BAC) that is considered within the legal limit, which is below 0.08%. Our highest ethanol percentage was 0.10% (v/v) ethanol, which would be considered just over the legal limit, resulted in 75.8% of the embryos presenting with abnormalities, compared to only 21% abnormalities in the 0.02% (v/v) ethanol treatment. These results illustrate that developmental effects of ethanol in urchin embryos are dose-dependent, which could allow further investigations into the impacts of consuming different amounts of alcohol during pregnancy. Based on the data obtained on the gut, we can also conclude there is a possible link between embryonic exposure to alcohol and gut abnormalities. These abnormalities of the gut caused by alcohol exposure are possibly contributing factors to the appearance of gastrointestinal reflux disease in humans, however more studies are required to understand this relationship.
Acknowledgements
We would like to thank Jongmin Nam, Ph.D. and Catherine Guay for assisting us throughout our experiment and thank Dr. Nam for providing the sea urchins to conduct our research.
References
Ernst, S.G. (2011). Offerings from an Urchin. Dev. Biol. 358, 285–294.
Gupta, K.K., Gupta, V.K., and Shirasaka, T. (2016). An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 40, 1594–1602.
McClay, D.R. (2011). Evolutionary crossroads in developmental biology: sea urchins. Development 138, 2639–2648.
Sujay, N.K., Jones, M., Whittle, E., Murphy, H., and Auth, M.K.H. (2012). Severe Gastrooesophageal Reflux Disease Associated with Foetal Alcohol Syndrome. Case Rep. Pediatr. 2012, 1–3.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
