Brittany N. Ruiz, Anthony R. Monte Carlo III, Melanie Lopez and Terra Hoskins
Department of Biology, Rutgers University, Camden, NJ. 08102
Abstract
The current opioid crisis calls for the exploration of non-pharmacological pain management strategies for chronic pain. Opioids, a standard therapeutic option for chronic pain, has harmful, addictive side effects. Social isolation is often another consequence of chronic pain. Social behavior and interactions improves mental health. Exploring the relationship between social behavior and chronic pain is thus imperative to create new therapeutic strategies to decrease opioid use. We propose chronic pain decreases social behavior in Drosophila Melanogaster and through the facilitation of social behavior, pain sensitivity will improve. We will study this by facilitating mating behavior of D. melanogaster as a measure of social behavior. Our first aim explores whether chronic pain reduces mating behavior by inducing neuropathic pain via leg amputation in adult male drosophila and subsequently measuring its impact on courtship behavior. Our second aim will determine if modulating social behavior in isolated drosophila changes thermal sensitivity. Together, these experiments will model for the first time in D. melanogaster the behavioral intersection between social behavior and pain.
Specific Aims
An estimated 50 million adults suffer from chronic pain in the US (Dahlhamer et al., 2018). Chronic pain includes months of persistent pain that can inhibit social activity and interaction (Milinkeviciute et al., 2012). Humans are social creatures and social isolation has a negative effect on physiologic and psychological functioning of human health (Bhatti and Haq). Unfortunately, there is not any one treatment that can relieve chronic pain for all people. The pathophysiology of pain is individualized. Chronic pain develops through biological factors such as an injury or a disease, psychological and social factors (Mackey, 2016). Opioids are currently used for managing chronic pain but can have harmful side effects. This causes strain on households and relationships and can induce further social isolation. To reduce the overreliance on opioids, non-pharmacological treatment strategies may be helpful.
There is a limited understanding behind the mechanisms of how social behavior affects pain, but likely functions through the amygdala, a neuronal structure involved in processing the emotional component of pain (Thompson and Neugebauer, 2017). Studies have found hyperactivity and increased synaptic transmissions of the amygdala in subjects experiencing chronic pain (Gonçalves et al., 2008; Thompson and Neugebauer, 2017). Other studies suggest an overlap of neural pathways responsible for experiencing physical and social pain (Sturgeon and Zautra, 2016). Chronic pain patients may isolate themselves, further increasing their social pain while experiencing physical pain. The increased neuroplasticity of complex amygdala neurons may be responsible for depressive symptoms in chronic pain patients. Further research is necessary to understand if decreased social behavior can impact physical pain.
Understanding the mechanisms that connect social behavior and pain is prerequisite to developing more effective therapies. Simple organisms like Drosophila melanogaster have naked-nerve endings that show resemblance to vertebrate nociceptors and have the ability to sense and respond to tissue damage (Milinkeviciute et al., 2012). D. melanogaster shares social behaviors reminiscent of complex animals’ social behaviors (Sokolowski, 2010). The plethora of genetic, social and pain research on D. melanogaster makes them a great organism to study from. Their simple social behaviors can be easily analyzed, and research describes they can experience chronic pain (Khuong et al., 2019; Nichols et al., 2012). By using D. melanogaster, we can easily and effectively conduct pain and social behavior studies. We hypothesize D. melanogaster with chronic pain will have decreased social behavior, but that social interaction will reduce said pain.
Specific Aim 1: Determine if chronic pain decreases social behavior in D. melanogaster. Using courtship behavior as a measure of social behavior, we hypothesize that D. melanogaster with chronic pain will show decreased courtship behavior as compared to controls. We will induce pain via a validated leg amputation.
Specific Aim 2: Determine whether facilitating social behavior can improve chronic pain in D. melanogaster. We will rear D. melanogaster with leg amputations in isolation or not in isolation and measure their pain sensitivity with a thermal nociceptive assay. We hypothesize that flies kept in isolation to be hyper-responsive to pain whereas flies who engage in social behavior will be less responsive to pain.
Significance
The sensation of pain is incredibly important to the survival of an organism; Without pain, the organism would be unable to detect harmful stimuli that could result in permanent damage to essential body structures. However, hypersensitivity to pain can be a disadvantage to an organism, and the long-term sensation of persistent pain can negatively impact that organism’s behavior. An appropriate amount of pain sensation is important to organismal survival, though the amount of pain experienced varies between individuals. For instance, injury increases pain sensitivity (Price and Dussor, 2014). This discovery can explain why chronic pain conditions exist, as organisms that are constantly in pain are more sensitive to it than others. Chronic pain can be caused by injuries and other disorders such as cancer, diabetes, and physical injury (Khuong et al., 2019). The onset of chronic pain can lead to a number of comorbidities, further worsening the pain one experiences, such as muscle degeneration due to lack of movement.
Chronic pain affects millions of people around the world, making significant impacts on people’s lives in both physical and social ways. It is estimated that nearly 50 million Americans struggle with some form of chronic pain (Dahlhamer et al., 2018). To preserve the wellbeing of millions of Americans, as well as millions around the world, it is imperative that scientists work diligently to understand the fundamental pathology of chronic pain so that we might develop the most effective ways to treat it. Understanding the fundamental neural mechanisms of pain and the changes in the body that they cause is a necessary step to develop alternative treatments for physical or social impairments.
The current treatment of chronic pain mostly involves the use of opioids which are incredibly addictive and easily abused. There are a number of dangerous side effects if an overdose occurs, most frequently being respiratory suppression leading to cardiac arrest and subsequent death. Drug tolerance is another important consideration in the treatment of chronic pain, as the consistent use of the drug over a period of time can result in decreased drug efficacy, making the need for alternative, effective, and less costly forms of treatment even greater. Similar to how genetics play a role in a person’s pain experience, genetics also play a role in the efficacy of certain drugs since each patient will respond differently to a drug.
In humans, chronic pain negatively impacts relationships and is the cause of a number of behavioral modifications in the home, including isolation, conflict with a romantic partner, feelings of anger and anxiety, and resentment towards family members (Forgeron et al., 2015; Snelling, 1994). Interestingly, the amount of familial coping mechanisms that were present within the home environment played a role in the prominence of these behaviors (Snelling, 1994). Thus, social behavior is impacted by chronic pain and that social activities could improve pain outcomes.
An extremely effective and useful tool in studying the genetic basis of many diseases in biological research is the fruit fly species D. melanogaster, due to their genetic similarities to humans, simplicity as an organism, short reproduction cycle, as well as the incredible amount of studies available on behavior and genetics (Greenspan and Ferveur, 2000; Pandey and Nichols, 2011). Along with this, the understanding of the neural pathways and mechanisms behind their display of courtship behavior is incredibly robust and well understood. D. melanogaster display pain behavior making it a valuable animal model for studying the pathophysiology of both acute and chronic pain (Khuong et al., 2019; Price and Dussor, 2014). Further, courtship assays can reliably be used to quantify the social behavior of the flies (Sokolowski, 2010). Together, this suggests a viable behavioral strategy to studying the intersection of pain and social behavior.
This suggests that an alternative or additional treatment for chronic pain might be offered by opportunities to socialize and cope with their pain in the presence of others. D. melanogaster is a viable model to study the intersection between chronic pain and social behavior. We plan to test this hypothesis by utilizing the robust behavior of fruit flies during courtship and the thermal nociceptive assay. We hypothesize that social isolation of flies experiencing chronic pain will result in a lower pain tolerance and worse performance in the thermal nociceptive assay.
Approach
Specific Aim 1: We hypothesize that D. melanogaster experiencing chronic pain will show decreased courtship behavior. We will measure the courtship behavior of male D. melanogaster through courtship assays (Nichols et al., 2012; Sokolowski, 2010). Newly emerged D. melanogaster will first be separated into two groups based on sex, male and female. It is imperative to separate the flies at the start of the experiment to ensure virgin flies do not mate.
Courtship assays will be held in a 3D printed mating wheel that holds three courtship chambers measuring 8×5 mm in length. This allows for three courtship assays to be run at the same time. Control groups contain a wild-type male and virgin female. We will induce chronic pain via a leg amputation (Fig. 1) since this surgery was previously validated and induces long term chronic pain without modifying locomotion (Khuong et al., 2019). We will anesthetize the flies by placing them on an ice block under a microscope (Pulver and Berni, 2012). By looking through a microscope we can successfully amputate the right middle leg at the femur segment (Khuong et al., 2019). Khuong et al. found no significant difference in mobility with amputated flies and non-amputated flies when assessing average movement speed (2019). The amputation should not have an effect on the experiment other than causing chronic pain in the flies.
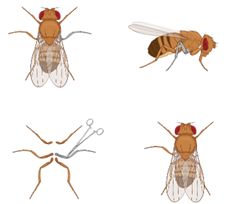
Figure 1. Schematic for amputation to induce chronic pain state. The right middle leg of amputate the right middle leg at the femur segment will be cut as previously described to induce a state of chronic pain.
To begin the courtship assays, we will introduce the virgin female fly to the courtship chamber before introducing the male fly (Nichols et al., 2012). We will record when each of the following behaviors occur during each courtship assay: “Orientation (the male orients towards the female), Tapping (the male taps the female), Wing song (the male extends and vibrates one wing), Licking (the male licks the female genitalia), Curling (the male curls its abdomen under itself) and Copulation attempt (Curling activity while attempting to mount the female)” and we will record the total time engaged in courtship behavior until copulation to calculate the courtship index (Nichols et al., 2012). The courtship index of wild type flies range between 0.6-0.8 (Nichols et al., 2012).
We will perform this courtship assay in two groups: 1) amputated male flies with non-amputated female flies and 2) non-amputated male flies with non-amputated female flies. Using a power analysis, we determined a sample size of 24 to be sufficient in determining statistical significance. We predict amputated male flies will have a lower courtship index than non-amputated male flies.
Specific Aim 2: We will determine whether isolation enhances pain in amputated D. melanogaster. We will prep the experimental male flies by amputating the right middle leg to induce chronic pain (Khuong et al., 2019). We will then house amputated flies in either isolation or in a group of nine amputated males. We will then conduct the thermal nociception assay that was previously validated (Im and Galko, 2012; Khuong et al., 2019) (Fig. 2).
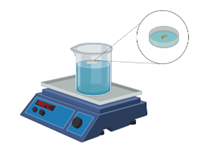
Figure 2. Schematic for measuring thermal sensitivity. Drosophila will be placed in a small plastic chamber and lowered onto a beaker of water set to a nociceptive temperature of 40°C. The time for all flies to move from the lower surface to the upper surface to escape the temperature will be used to assess thermal sensitivity.
Each fly will be placed in a covered petri dish on a water bath that is at a comfortable temperature of 25°C on a hot plate (Im and Galko, 2012). We will use the hot plate to ensure that the water will heat gradually to the noxious temperature of 40°C (Im and Galko, 2012). We will record how many seconds the fly remains on the 40°C noxious surface before flying to the top of the covered petri dish. The latency to jumping off the noxious surface is a measure of sensitivity to a thermal stimuli. Previous studies have demonstrated that amputated animals jump from the surface with lower latencies than non-amputated animals. If our proposed hypothesis is supported, then the isolated animals should have a lower latency (increased sensitivity) than the non-isolated animals. A power analysis suggests that groups of 40 flies would be sufficient.
Broader Impacts
Conventional medications for chronic pain include nerve blockers, analgesics, and opioids. Opioids are effective at treating short-term pain, but there is not much evidence of its effectiveness in treating chronic pain in patients. This lack of evidence has not stopped doctors from prescribing opioids to their patients, leading to misuse, addiction, overdose and in extreme cases, death. Studying chronic pain in D. melanogaster can lead to a better understanding of chronic pain in humans and can lead to the discovery of new, non-addictive treatments.
The COVID-19 pandemic is forcing people, all over the world into isolation as a way to prevent the spread of the virus. Pain centers are closed, and doctors have to find alternatives to treat their patients. Chronic pain patients are not able to get the assessments and treatments they need, leading to the worsening of their condition. People waiting for assessment often report severe levels of pain that interfere with their ability to function, and reports of severe pain are associated with more severe levels of depression in 50% of chronic pain patients (Eccleston et al., 2020). To remotely support management of pain patients, healthcare providers are turning to telemedicine, electronic health, mobile health, and virtual reality augmented reality, digital therapeutics, remote treatment, and remote therapy. Delivering pain self-management has been shown to be effective in some cases. However, many of the behavioral components of eHealth self-management are not only potentially helpful for managing pain but also for emotional distress related to the COVID-19 pandemic (Eccleston et al., 2020).
Increasing our knowledge of the factors and mechanisms of chronic pain will allow for doctors to have an improved understanding of caring for chronic pain patients. This increased knowledge would also improve surgeries, interventional procedures, medications, psychology interventions, physical therapy, and other complementary approaches (Mackey, 2016). A greater appreciation will come for an interdisciplinary approach to optimize pain care, especially in more complicated cases. Doctors should take into consideration a patient’s current social environment, such as if the patient is still socializing with their family and friends. If they are not, doctors should list that as one of the steps they want their patients to take on before seeing them again. In addition to patients going to physical therapy, they should also go to social therapies where others who experience chronic pain can come together to support one another as well as participate in different activities together. Chronic pain patients socializing with one another will provide support, understanding, and empathy that they may not be experiencing through their other social environments. As more efficacious treatments are identified, opioids will most likely continue to move down the list of approaches.
References
Bhatti, A.B., and Haq, A. ul The Pathophysiology of Perceived Social Isolation: Effects on Health and Mortality. Cureus 9.
Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., Kerns, R., Von Korff, M., Porter, L., and Helmick, C. (2018). Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67, 1001–1006.
Eccleston, C., Blyth, F.M., Dear, B.F., Fisher, E.A., Keefe, F.J., Lynch, M.E., Palermo, T.M., Reid, M.C., and Williams, A.C. de C. (2020). Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. PAIN 161, 889–893.
Forgeron, P.A., King, S., Stinson, J.N., McGrath, P.J., MacDonald, A.J., and Chambers, C.T. (15). Social Functioning and Peer Relationships in Children and Adolescents with Chronic Pain: A Systematic Review (Hindawi).
Gonçalves, L., Silva, R., Pinto-Ribeiro, F., Pêgo, J.M., Bessa, J.M., Pertovaara, A., Sousa, N., and Almeida, A. (2008). Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp. Neurol. 213, 48–56.
Greenspan, R.J., and Ferveur, J.-F. (2000). Courtship in Drosophila. Annu. Rev. Genet. 34, 205–232.
Im, S.H., and Galko, M.J. (2012). Pokes, Sunburn, and Hot Sauce: Drosophila as an Emerging Model for the Biology of Nociception. Dev. Dyn. 241, 16–26.
Khuong, T.M., Wang, Q.-P., Manion, J., Oyston, L.J., Lau, M.-T., Towler, H., Lin, Y.Q., and Neely, G.G. (2019). Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila. Sci. Adv. 5, eaaw4099.
Mackey, S. (2016). Future Directions for Pain Management: Lessons from the Institute of Medicine Pain Report and the National Pain Strategy. Hand Clin. 32, 91–98.
Mellado, B.H., Falcone, A.C.M., Poli-Neto, O.B., Rosa e Silva, J.C., Nogueira, A.A., and Candido-dos-Reis, F.J. (2016). Social isolation in women with endometriosis and chronic pelvic pain. Int. J. Gynecol. Obstet. 133, 199–201.
Milinkeviciute, G., Gentile, C., and Neely, G.G. (2012). Drosophila as a tool for studying the conserved genetics of pain. Clin. Genet. 82, 359–366.
Nichols, C.D., Becnel, J., and Pandey, U.B. (2012). Methods to Assay Drosophila Behavior. JoVE J. Vis. Exp. e3795.
Pandey, U.B., and Nichols, C.D. (2011). Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 63, 411–436.
Price, T.J., and Dussor, G. (2014). Evolution: The Advantage of ‘Maladaptive’ Pain Plasticity. Curr. Biol. CB 24, R384–R386.
Pulver, S.R., and Berni, J. (2012). The Fundamentals of Flying: Simple and Inexpensive Strategies for Employing Drosophila Genetics in Neuroscience Teaching Laboratories. J. Undergrad. Neurosci. Educ. 11, A139–A148.
Seeman, T.E. (1996). Social ties and health: The benefits of social integration. Ann. Epidemiol. 6, 442–451.
Snelling, J. (1994). The effect of chronic pain on the family unit. J. Adv. Nurs. 19, 543–551.
Sokolowski, M.B. (2010). Social Interactions in “Simple” Model Systems. Neuron 65, 780–794.
Sturgeon, J.A., and Zautra, A.J. (2016). Social pain and physical pain: shared paths to resilience. Pain Manag. 6, 63–74.
Thompson, J.M., and Neugebauer, V. (2017). Amygdala Plasticity and Pain (Hindawi).
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
