Lauren Owens, Hoi Tran, Isabel Garcia, Vishruti Patel, Elizabeth Hardy
Department of Biology, Rutgers University, Camden NJ 08102
Abstract
The effects of chronic social isolation play an important role in health and development, as a loss of social interaction can lead to an increased risk of mortality and cardiovascular disease. In male Drosophila melanogaster, chronic isolation reduces the likelihood of successful copulation as compared to non-isolated males. However, the role of chronic social isolation in female mating response remains unknown. Males and females have exhibited varied responses to social stressors, as females displayed an increased sensitivity. Here we show that the behavioral effects of chronic social isolation on females differ from those observed by males in recent studies. Our results suggest that chronic isolation has no effect on courtship acceptance or rejection behaviors in females. Compared to the non-isolated negative controls, there is no significant difference in the latency to copulation and other mating behaviors exhibited by the isolated females. This suggests that the effect of isolation on female courtship contrasts the male behavior. Examining the prevalence of similar effects across a broader range of species could provide more valuable insight into the sex-specific significance of social isolation.
Introduction
During the peak of the COVID-19 pandemic, social isolation was at an all-time high (Carotta et al., 2022). While these safety measures were essential, there may have been unintended consequences on human health according to the biopsychosocial aspects of disease (Umberson and Montez, 2010). From a physical perspective, research has shown that self-rated health or risk of mortality from cardiovascular disease was seen more frequently in countries where people are more independent (Okely et al., 2018). This failure to interact with others can prevent the formation and fostering of relationships often necessary for development and reproduction. Therefore, the increase in isolation has been correlated with a decrease in marriage and a subsequent decrease in courtship and mating behaviors (Wagner et al., 2020). Social isolation has various effects physically, psychologically, and behaviorally. Therefore, it is crucial to study the toll that isolation can have.
It is evident that social isolation affects many species and can have detrimental consequences for health. One study demonstrated the effects of chronic isolation in animals, including sleep loss and overconsumption of food (Li et al., 2021). Chronic isolation in Drosophila melanogaster is defined as a period of seven days, as the lifespan and development period for this species is so limited (Li et al., 2021). The response following social isolation on courtship behaviors has already been studied in males where researchers found that mating behaviors in non-isolated D. melanogaster were more successful than those in isolated males (Vora et al., 2022). Although the effect of chronic isolation on courtship success has been identified in males, similar research has yet to be studied in female D. melanogaster. Studies have revealed that females display increased sensitivity to social stress than males (Senst et al., 2016). This varying response further indicates that females from different species may react differently to social isolation than males (Senst et al., 2016). These observed differences in sensitivity in response to social isolation among males and females are reasons to study female D. melanogaster. Additionally, this is an important aspect to further explore because females play a different role in the mating process than males. They have the responsibility of accepting or rejecting the males’ courting attempts.
The method we chose for characterizing new interactions following the period of isolation forfemale D. melanogaster was by assessing their rejection behaviors. Rejection can be exhibited in several ways by female D. melanogaster. These behaviors involve the closing of the wings during copulation, kicking of the legs, or pushing out her ovipositor (Wang et al., 2020). There is the possibility that females would be more willing to mate and accept these advances after only ever experiencing isolation. On the other hand, the females could behave similarly to what was previously observed in males, leading to unsuccessful copulation by rejecting the attempts of their mate. Research shows that isolation has behavioral consequences and causes a decline in male mating behavior. Thus, studying the female D. melanogaster mating response will lead to knowledge of how social isolation differs in males versus females.
When making observations about possible human responses to isolation, D. melanogaster is a useful experimental organism because studying behaviors in fruit flies serves as a tool that can be used to make connections to similar responses in humans. (Vora et al., 2022). D. melanogaster is a prime model for research because the species shares similarities between their fly homologs and human genes. They contain molecular, genetic, and behavioral tools to uncover mechanisms underlying social isolation-induced effects (Vora et al., 2022). The size of D. melanogaster makes them easy to manipulate and isolate for experimental purposes. This ensures that their behaviors can be recorded easily on a small scale. It is also worth noting that the strain D. melanogaster is readily available and preferred for many other reasons. The short lifespan of D. melanogaster grants plenty of time to obtain results. D. melanogaster readily copulates, granting the ability to obtain a larger population size to study. This continued reproduction of larvae is an integral part of the experimental process that must occur to obtain replicates. For this study, the effects of social isolation on the model system, D. melanogaster, will be comparative to human-induced isolation effects, such as throughout the COVID-19 pandemic.
In our current study, we used an experimental organism, D. melanogaster, to test the hypothesis that the induced social isolation of females will increase the rejection response to courting males. This hypothesis is made on the basis that isolation can alter behavioral responses in D. melanogaster to increase aggression and loneliness and, therefore, decrease the willingness to mate with the newly introduced males. Testing this hypothesis involved reintroducing the chronically isolated females to a male counterpart and observing their response over the span of ten minutes to measure the female-specific courtship index which consisted of percent success, latency to copulation, and the frequency of mating behaviors observed in female D. melanogaster. We find that isolation of females had no noticeable effect on courtship behavior, providing further insight into the sex-specific impact of isolation in D. melanogaster.
Materials & Methods
Fly Stock
The strain used in this experiment is the Oregon-R wild type purchased from Bloomington Drosophila Stock Center. All flies in the current study were stored at room temperature with fresh food. This food mixture consisted of purified water, agar, potassium sugar, calcium chloride, sucrose, dextrose, deactivated yeast, and corn. New food was provided weekly to ensure the longevity of the fly lifespan and to grant the ability for the flies to reproduce.
Experimental Design
In order to test our hypothesis, we developed an experimental design to study the effects of chronic isolation on the mating response in female wild-type D. melanogaster. The first step in the design involves the collection of virgin female flies. Virgin females were obtained within four hours of hatching to ensure they were virgin D. melanogaster. This meant assigning someone to check on our reproductive vials at certain time points every day. If hatching had occurred, the females and males were separated into their respective vials (Figure 1). Observing the flies under the microscope confirmed that they were virgins before being relocated.
The negative control group consisted of virgin females (n = 15) that were grouped together and housed in a vial in order to avoid chronic isolation, while ensuring they would still readily mate. Virgin female D. melanogaster under 48 hours old are sexually immature and will not mate (Ishimoto and Kamikouchi, 2020). To ensure the sexual maturity of the virgin group-house female D. melanogaster and willingness to mate in each trial, we used five-six day old female D.melanogaster. The positive control group consisted of females (n = 15) that had been recently mated within five days (Aranha and Vasconcelos, 2018). These females were mated to represent a non-receptive state in the rejection assay (Balaban-Feld and Valone, 2018). The experimental group involved virgin females (n = 15) that were collected and separated into their own isolated vials after hatching. Each of these females were isolated in a vial for a seven-day period, which is considered a period of chronic social isolation (Li et al., 2021). Each trial was run consecutively at the same time, between 5:00-8:00 pm, over several weeks to avoid variability amongst each group. Adding the flies to the chamber involves using a fly aspirator tube (Figure 1) for transport, without anesthesia. When running the trials, one female will be placed into the mating chamber (Figure 1) and allowed to acclimate for ten minutes. Then the male D. melanogaster is introduced to the chamber for analysis to begin across a ten-minute courtship index period. The latency to copulation is recorded and capped after a ten-minute period. Additionally, the frequency of certain mating behaviors were measured. The acceptance behavior includes vaginal plate opening and rejection behaviors include kicking and ovipositor extrusion.
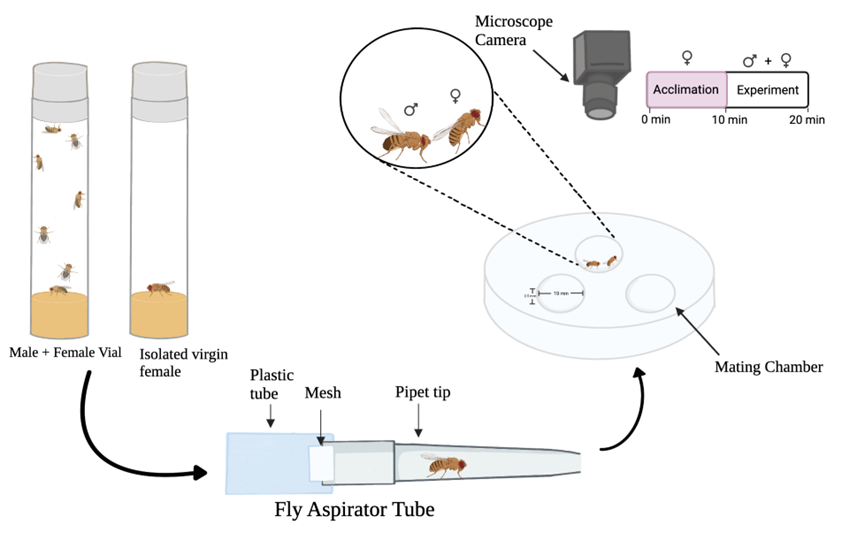
Figure 1. Illustration of experimental design. There are two vials, one male and female group housed and one virgin female isolated. A fly aspirator tube is used to transfer the files from the vial to the mating chamber. The design of the experiment involves one female fly being placed in the mating chamber for an acclimation period of 10 minutes, followed by one male fly being placed in the mating chamber. The mating chamber is recorded with a microscope camera for 10 minutes. These videos are then analyzed for courtship behaviors and latency to copulation.
Statistical Analysis
For our statistical analysis, we performed a one-way ANOVA with a Tukey post hoc in R statistical software (v4.2.2; R Core Team 2022) in order to determine if there are any significant differences in the total number of mating behaviors between the experimental and control groups. For latency to copulation, we performed a t-test in R statistical software (v4.2.2; R Core Team 2022) in order to determine if there is a significant difference between the experimental and negative control group.
Mating Chamber
The mating chambers are 3D-printed wheels with three chambers each with the dimensions of 10 x 2.5 mm. A clear, plastic cover slip keeps the flies in their chamber for observation. After the female and male are introduced to the chambers, the latency of copulation and rejection behaviors of females over a 10-minute period are observed with a microscope. Videos were captured using Photoron FASTCAM Viewer software (PFV4) and analyzed by the same researchers across all groups to ensure consistency in the specific rejection or acceptance behaviors observed.
Mating Behavior Assay
After the introduction to the mating chamber, the number of acceptance and rejection behaviors will be measured. The female will be introduced to the mating chamber first. There is a ten-minute acclimation period before the male is entered to the chamber. Then the mating behavior assay is recorded as the amount of times that a female performs the certain acceptance or rejection behavior towards the male over a 10-minute interval. The behaviors associated with female acceptance are vaginal plate opening and successful copulation. The behaviors associated with female rejection are outlined as closing of the kicking of the legs or pushing out her ovipositor (Wang et al., 2020).
Latency to Copulation
The latency to copulation is measured using the courtship index, which covers a span of ten minutes, after introduction to the chamber. After the acclimation period, the D. melanogaster male orienting himself towards the female is the first courtship behavior. This signifies that the male is attempting to mate. The latency to copulation is the amount of time from the D. melanogaster male’s initial introduction into the mating chamber, until copulation occurs (Nichols et al., 2012). The copulation indicates that the male is successful in his courtship attempt and not rejected by the female. The latency to copulation is capped at ten minutes as this is the period for courtship index. Females that failed to copulate are not included in the latency to copulation results.
Results
To test the hypothesis that isolation would decrease receptivity, we isolated virgin female for seven days (Figure 1). The frequency of different mating behaviors, percent success, and latency to copulation are recorded over a ten-minute period, known as the courtship index.
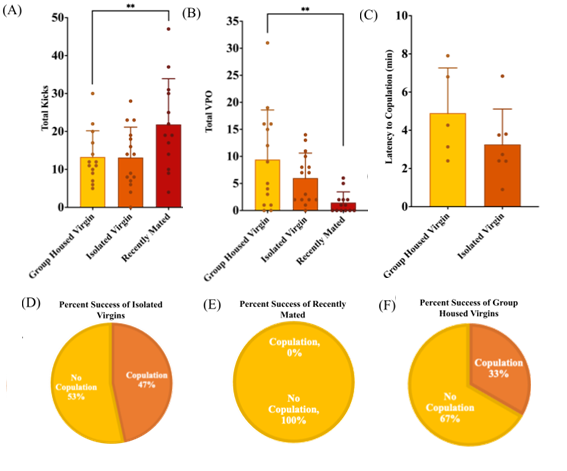
Figure 2. Effects of female isolation on mating response. D. melanogaster are split into three groups: group-housed virgin females, isolated virgin females, and recently mated females. Error bars represent standard deviation (SD) ; (A) Total kicks observed in 10 minute mating assay across all groups ; (B) Total Vaginal Plate Openings observed in 10 minute mating assay across all groups ; (C) Latency to copulation (D-F) Percent success with 100% indicating copulation within 10 minutes and 0% indicating failure to copulate within 10 minutes.
Kicking is a rejection behavior exhibited when the female extends one of her legs to push the male away. We found that the group housed virgin females had a mean (±SD) total number of kicks of 13.286 ± 6.90. This number was significantly lower than the recently mated females with a mean total number of kicks of 21.846 ±9.537. These results indicate a significant increase in kicking behavior frequency in recently mated females compared to group housed virgin females (ANOVA, p<0.05, Figure 2A). The recently mated are expected to have an increased number of kicks due to its association of rejection behavior. This significance confirms the validity of our experimental design. Recently mated females should be completely unreceptive and exhibit the most rejection behaviors. Isolated virgin females had a mean total number of kicks of 13.133 ±7.20. Based on our hypothesis, isolated females are expected to have an increase in kicking frequency as a result of the unwillingness to mate compared to the group housed females. However, no significance was shown between the isolated virgin females and the non-isolated virgin females (ANOVA, p>0.05, Figure 2A). This indicates that isolation had no effect on the rejection behavior of kicking.
Vaginal plate opening (VPO) is an acceptance behavior exhibited by female D. melanogaster. In response to the male courtship song, the female can be seen opening up her vaginal plate in order to signal receptiveness to mate (Wang et al., 2020). We found that the group housed virgin females had a mean (± SD) total number of VPOs of 9.429 ±9.163. Based on the hypothesis, it is expected that group housed virgin females would exhibit these acceptance behaviors as a signal that they are ready to mate. However, there is a large amount of variability in the number of VPOs observed. This indicates that other factors may contribute to the willingness of the female to mate. Recently mated females had a mean frequency of VPOs of 1.462 ±6.483. These results indicate a significant decrease in VPO behavior frequency in recently mated females compared to non-isolated virgin females (ANOVA, p<0.05, Figure 2B). This is expected as VPO is an acceptance behavior and is typically exhibited by recently mated females at a much smaller rate. Isolated virgin females had a mean total number of vaginal plate openings of 6.0 ±7.023. However, no significance was shown between the isolated virgin females and the non-isolated virgin females (ANOVA, p>0.05, Figure 2B). There is great variability among the group, indicating that there is a need for more replicates.
Ovipositor extrusion is a rejection behavior that is only exhibited by recently mated females. This behavior involves a clear extrusion of the ovipositor out of the female, indicating an unwillingness to mate (Wang et al., 2020). We found that the group housed virgin females had a mean (± SD) frequency of ovipositor extrusions of 0 ±0. Recently mated females had a mean frequency of ovipositor extrusions of 3.538 ±2.591. Isolated virgin females had a mean frequency of ovipositor extrusions of 0 ±0. The results show a significant increase in ovipositor extrusions by the recently mated group as compared to the negative control group and experimental group (ANOVA, p<0.05). This is expected as ovipositor extrusion is a rejection behavior that is exhibited by non receptive females, but not by virgin females. This is important to include as it confirms that our experimental design was a good indication of the different mating behaviors performed by females.
Success is measured as 100% for copulation occurring within 10 minutes and 0% for failure to copulate within 10 minutes. The cap of 10 minutes was made based on the courtship index for D. melanogaster. Group housed virgin females exhibited a percent success of 33.33%. Isolated virgin females exhibited a percent success of 46.67%. Recently mated females exhibited a percent success of 0%. No statistics are necessary for these percentage results. However, they are a good comparison to indicate the rate of copulation among all groups. The percent success is also a confirmation of the positive control being representative of total rejection.
Latency to copulation is measured from the time the male is introduced to the mating chamber until copulation is successful. For the purpose of this experiment, only successful copulations are considered in the latency to copulation results. We found that the group housed virgin females had a mean latency to copulation (± SD) of 4.9 minutes ±1.06. We found that isolated virgin females had a mean latency to copulation (± SD) of 3.26 minutes ±0.70. A latency to copulation of 10 minutes indicates rejection. Recently mated females were unable to copulate within 10 minutes for every replicate. The recently mated females are expected to exhibit the highest latency as they fail to copulate in the unreceptive state. However, the results for latency to copulation were insignificant across between the group housed and isolated virgin females (t-test, p>0.05, Figure 2C). This indicates that isolation has no effect on latency to copulation in female D. melanogaster.
Taken together, we concluded that isolation does not lead to a significant increase in acceptance or rejection behaviors. In addition, isolation does not lead to a significant decrease in latency to copulation as predicted. Chronically isolated virgin female D. melanogaster display a similar number of rejection and acceptance associated behaviors compared to the group housed virgins females (Figure 2A & 2B). The latency to copulation (Figure 2C) among the group housed versus isolated virgin females are similar. The significance between group housed virgin females and recently mated females for mating behaviors and latency to copulation, as well as a percent success of 0% indicates the validity of the controls chosen.
Discussion
In our current study, we tested the hypothesis that chronically isolated females will have increased courtship rejection behaviors. However, there were no significant difference in isolated versus non-isolated (group housed) females in their number of mating behaviors. We observed that the non-isolated females displayed a similar frequency of rejection and acceptance behaviors to the isolated experimental group. The non-isolated females also had a latency to copulation that was not significantly different from the isolated females. These results lead us to reject our original hypothesis and determine that chronic isolation has no effect on courtship behaviors in female D. melanogaster. While our study reveals no effect of isolation on female courtship behavior, it confirms that female response varies from previously observed male behaviors. Male isolation is shown to decrease courtship success in other studies, as opposed to the female isolation in the current study that shows no significance (Vora et al., 2022). This data, in comparison to other available literature, is successful in revealing that males and females have different behavioral responses to chronic isolation.
Successful courtship is essential for the reproduction of many species. From an evolutionary perspective, courtship can be seen as a series of adaptations to ensure successful copulation between the male and female. The process of courtship is different between the male and female D. melanogaster. For males, courting behaviors involve pursuing the female of interest and trying to ensure successful copulation. Courtship is initiated by the male approaching the female and engaging in a series of behaviors such as courtship song, wing extension, and tapping. Female courting behaviors involve the decision to accept or reject the male’s courting attempts. The females will make the decision to either accept or reject the male’s advances based on visual signals, pheromones, and genetic compatibility (Wang et al., 2020). In a study on the HPA axis responses, men are shown to have higher cortisol release during achievement-based challenges, while women have higher cortisol release during rejection-based challenges (Uhart et al., 2006). This reveals that the fear of rejection is more stressful for women, while men are more anxious about achieving their goal. If women are more likely to avoid rejection, it is possible that this could have an effect on their mating behaviors. Anxiety and stress about rejecting the male will prevent the female from exhibiting a higher rejection response. Female behaviors display their receptivity to copulation, or lack thereof. Therefore, the acceptance and rejection behaviors of the females should demonstrate to the male their level of willingness to copulate. It is possible that the factors leading to acceptance or rejection behaviors are based more on the level of sexual maturity and mating status of the female, rather than social influence.
Our study suggests that a female displaying a lack of receptivity will not impact the male from pursuing copulation. Because the male’s ultimate goal is successful copulation, rejection from the female may not always stop the male from continuing to attempt to court. The number of acceptance or rejection behaviors displayed by the female could be in direct correlation to the amount of courtship persistence displayed by the male. A possible explanation for the lack of influence of chronic isolation in females could be because of this different role in courtship. Their acceptance and rejection behaviors could have more to do with the amount or quality of the male’s attempts, as opposed to the previous social environment. The varying duties of males and females in courtship suggests that males are more responsible for determining if copulation will be successful, as the female responsibility relies completely on responding to the males.
Comparisons between the isolated and non-isolated female groups show no significant differences in the observed frequency of mating behaviors. Therefore, female flies appear to be less influenced by social isolation than their male counterparts who decrease courtship behaviors when isolated. The results of this study indicate that females’ ability to copulate does not depend on previous social interaction. Lower social cues in females can still allow them to experience a response or effect. When it comes to their mating behavior, it is possible that females possess a higher threshold for social stimulation. Isolation may have not affected females at a period of seven days because of this higher threshold. Perhaps the chronic isolation period is longer in females, and a greater effect could have been observed had we isolated the females longer. In the context of the mating behavior of female flies, having a higher threshold for social stimulation suggests that female flies may not require prior social interaction to initiate and maintain their mating behavior compared to males.
One major area of variability that could have led to insignificant data is the age of the flies utilized. While the chronic isolation period was standardized to seven days, the flies were born at different times and the age could have contributed to the mating behaviors. The variability of ages among the flies could have also led to different levels of sexual maturity that could affect the mating response. The time of day could have also been a factor that affected the data. The circadian rhythm of D.melanogaster leads to an energy peak in the evening (Collins et al., 2004). The experiments were run between 5:00-8:00 pm every day. This timeline could have caused an increase in activity and mating behaviors. A final source of error could be that our experiment was underpowered. A power analysis based off our variability and those within the literature suggests each group would need 79 replicates, but due to limited time throughout the experiemntal time window, our study only had 15 in each group.
Future studies could make stronger conclusions if including hormonal and neural control analysis that may affect the female mating response to social isolation. Hormonal levels are influenced by social interactions and contribute to differences between males and females (Nelson, 2023). These studies could also be expanded to other species that may be impacted by isolation differently than Drosophila. Nevertheless, our study provides some insight into the sex-specific impact of social isolation on courtship behaviors.
Acknowledgements
We would like to thank Dr. Kwangwon Lee and Dr. Nathan T. Fried for their continued support through our project. We would also like to thank the Rutgers Camden Biology Department for their facilities and the opportunity to bring this project to fruition.
References
Aranha, M.M., and Vasconcelos, M.L. (2018). Deciphering Drosophila female innate behaviors. Curr. Opin. Neurobiol. 52, 139–148. https://doi.org/10.1016/j.conb.2018.06.005.
Balaban-Feld, J., and Valone, T.J. (2018). Changes in courtship behaviour following rejection: The influence of female phenotype in Drosophila melanogaster. Ethology 124, 149–154. https://doi.org/10.1111/eth.12715.
Carotta, C.L., Lavender-Stott, E.S., Garcia, A.S., and Liu, H.-L. (Stella) (2022). Relationship Status and Well-Being in the Context of the COVID-19 Pandemic. J. Fam. Issues 0192513X221105242. https://doi.org/10.1177/0192513X221105242.
Collins, B.H., Rosato, E., and Kyriacou, C.P. (2004). Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc. Natl. Acad. Sci. 101, 1945–1950. https://doi.org/10.1073/pnas.0308240100.
Ishimoto, H., and Kamikouchi, A. (2020). A Feedforward Circuit Regulates Action Selection of Pre-mating Courtship Behavior in Female Drosophila. Curr. Biol. 30, 396-407.e4. https://doi.org/10.1016/j.cub.2019.11.065.
Li, W., Wang, Z., Syed, S., Lyu, C., Lincoln, S., O’Neil, J., Nguyen, A.D., Feng, I., and Young, M.W. (2021). Chronic social isolation signals starvation and reduces sleep in Drosophila. Nature 597, 239–244. https://doi.org/10.1038/s41586-021-03837-0.
Nelson, R. (2023). Hormones & Behavior. Noba. https://nobaproject.com/modules/hormones-behavior
Nichols, C.D., Becnel, J., and Pandey, U.B. (2012). Methods to Assay Drosophila Behavior. J. Vis. Exp. JoVE 3795. https://doi.org/10.3791/3795.
Okely, J.A., Weiss, A., and Gale, C.R. (2018). The interaction between individualism and wellbeing in predicting mortality: Survey of Health Ageing and Retirement in Europe. J. Behav. Med. 41, 1–11. https://doi.org/10.1007/s10865-017-9871-x.
Senst, L., Baimoukhametova, D., Sterley, T.-L., and Bains, J.S. (2016). Sexually dimorphic neuronal responses to social isolation. ELife 5, e18726. https://doi.org/10.7554/eLife.18726.
Uhart, M., Chong, R.Y., Oswald, L., Lin, P.-I., and Wand, G.S. (2006). Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31, 642–652. https://doi.org/10.1016/j.psyneuen.2006.02.003.
Umberson, D., and Montez, J.K. (2010). Social Relationships and Health: A Flashpoint for Health Policy. J. Health Soc. Behav. 51, S54–S66. https://doi.org/10.1177/0022146510383501.
Vora, A., Nguyen, A.D., Spicer, C., and Li, W. (2022). The impact of social isolation on health and behavior in Drosophila melanogaster and beyond. Brain Sci. Adv. 8, 183–196. https://doi.org/10.26599/BSA.2022.9050016.
Wagner, B.G., Choi, K.H., and Cohen, P.N. (2020). Decline in Marriage Associated with the COVID-19 Pandemic in the United States. Socius 6, 2378023120980328. https://doi.org/10.1177/2378023120980328.
Wang, F., Wang, K., Forknall, N., Parekh, R., and Dickson, B.J. (2020). Circuit and Behavioral Mechanisms of Sexual Rejection by Drosophila Females. Curr. Biol. 30, 3749-3760.e3. https://doi.org/10.1016/j.cub.2020.07.083.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International L
