Francis A. Fahning
Department of Biology, Rutgers University, Camden NJ 08102
Abstract
The circadian clock is a necessary component for the regulation of daily cellular activities. Previous studies have shown that pharmaceutical chemicals can alter the period of the circadian rhythm of certain organisms. This study was constructed to determine if the circadian rhythm of Neurospora crassa, a filamentous fungus and a successful eukaryotic model organism, could be altered through the use of chemicals that are easily accessible. The successful outcome could open avenues of developing drugs that would modify the circadian rhythms of eukaryotic organisms including humans.
Introduction
The circadian rhythm is an entrainable cyclic biological process with a period of about 24 hours. Another characteristic of the circadian rhythm is temperature compensation, meaning that the circadian rhythm will not change when an organism is exposed to an environment of the physiological range of temperatures. Neurospora crassa is an excellent organism in characterizing molecular mechanisms of the circadian rhythm (Baker 2012). N. crassa is classified as a filamentous fungus which grows in a web like formation. Its usual habitat is in the sub-‐tropical region and found on dead or decaying plant material often after a forest fire. N. crassa is an ideal candidate for research as its haploid life cycle allows for simple tracking when used for genetic modification.
Circadian rhythms are generated by a circadian oscillator, which involves transcription and translation of clock genes in a cyclic process. This process is organized of coupled negative and positive feedback loops. Specific to N. crassa is the frequency gene (frq) and white collar 1 (wc1) and white collar 2 (wc2) genes associated with the white collar complex (WCC). Within N. crassa’s circadian oscillator, frq influences the period of the rhythm (Baker 2012). The gene expression of frq is regulated by wc1 and wc2. WCC binds to the frq promoter and up-‐regulates the expression of frq in the early morning, a positive feedback loop. The protein FRQ begins and forms homodimmers to enter the cell nucleus to repress frq transcription by inactivating WCC, a negative feedback loop. WCC is then removed from the nucleus and initiates the negative feedback loop beginning in mid-‐day to late-‐day (Baker 2012). Following the reduction of WCC, frq levels go down and the synthesis of FRQ decreases.
Recent study showed that there is a metabolic oscillator whose function is not dependent on transcription or translation (O’Neill 2011). Another important finding of the same study was that pharmaceutical chemicals would influence the metabolic oscillator of O. tauri (a single cell organism) the same way as they would on that of human cells.
In this study we will test if the environmental chemicals influence the N. crassa clock gene frq. We hypothesized that N. crassa’s circadian rhythm would remain in a cyclic pattern with the chemicals having no affect. The significance of this experiment will ascertain if chemicals can influence the circadian rhythm and how great of an effect the chemicals can produce. The chemicals used are common to everyday people and may produce results beneficial for our knowledge as eukaryotic circadian oscillators operate in comparable fashions.
Materials & Methods
The experimental design of testing N. crassa was similar to the study performed on O. tauri (O’Neill 2011). This unique experiment consisted of testing a strain of N. crassa’s frq gene as it is the clock gene that influences the period length of N. crassa’s circadian rhythm. The frq gene was composed with a suspension of high glucose media and a luciferase bioluminescence reporter along with the administration of a drug/chemical. The strain was designated N. crassa frqlv2 luciferase. The luciferase reporter adheres to the mRNA transcriptional components of the frq gene and allows the ability to track protein synthesis.
Six conditions were used in the form of common chemicals containing stimulant properties. The purpose was to prove the hypothesis wrong and I believed the best choice was to illustrate this through the use of well known stimulants. The six chemicals/conditions used were pseudoephedrine, phenylephrine, two cigarette brands containing nicotine and various other chemicals, caffeine, and coffee.
|
Pseudoephedrine, primarily a nasal decongestant, is also classified as a stimulant and ergogenic aid decreasing the effects of fatigue and has been widely used by athletes (Gillies 1996; Hodges 2006). When compared to pseudoephedrine, phenylephrine (a substitute nasal decongestant) is to have a reduced effect on the adrenergic receptors of the sympathetic nervous system in mammals and both chemicals may show a differing result when administered to N. crassa. Nicotine is mainly absorbed through tobacco products and has stimulant properties.
The two tobacco products used in this experiment were Pall Mall cigarettes (containing additives) and Natural American Spirit Organic cigarettes (organically grown and without additives). Several other chemicals known as additives are contained within a cigarette. These chemicals have been approved by the FDA as food additives and may attribute to a variety of unpredictable effects. Caffeine is a well-‐known stimulant used to combat fatigue and can be found in beverages and pill form. The methods of caffeine administration for the experiment consisted of caffeine in tablet form and caffeine through coffee. Caffeine and coffee were compared as coffee contains chemicals such as monoamine oxidase inhibitors, which possess anti-‐depressant qualities, and β-‐carbolines norharman and harman, which are alkaloids which may produce psychoactive effects (Herraiz 2006).
The experimental set-‐up was as follows:
Pseudoephedrine: One 30mg tablet crushed with a mortar and pestle into a powder. Pseudoephedrine powder mixed into approximately 50ml of water at 30°C. Chemical powder sat for thirty minutes to dissolve.
Phenylephrine: One 10mg tablet crushed with a mortar and pestle into a powder. Pseudoephedrine powder mixed into approximately 50ml of water at 30°C. Chemical powder sat for thirty minutes to dissolve.
Caffeine: One 200mg tablet crushed with a mortar and pestle into a powder. Pseudoephedrine powder mixed into approximately 50ml of water at 30°C. Chemical powder sat for thirty minutes to dissolve.
Coffee: Approx. 25ml of Starbuck’s Pike’s Place Medium Roast coffee was obtained directly.
Pall Mall cigarette: The tobacco contents of one cigarette were removed and placed in a “cheese-‐cloth” type fabric. Approximately 50ml of water was raised to boiling temperature and the tobacco was added to create a solution.
Natural American Spirit Organic cigarette: The tobacco contents of one cigarette were removed and placed in a “cheese-‐cloth” type fabric. Approximately 50ml of water was raised to boiling temperature and the tobacco was added to create a solution.
200µl of 2% high glucose media was prepared for six replicates of each condition. Each replicate (pseudoephedrine, phenylephrine, Pall Mall cigarettes, Natural American Spirit cigarettes, caffeine, & coffee) was titrated with 200µl of media and 200µl of the chemical giving the 1:1 (full strength) ratio. A high glucose media was produced to provide nutrients for the N. crassa frqlv2 luciferase strain. The mRNA transcriptional bioluminescent reporter luciferin was also added along with the strain to track frq gene transcription levels. A 12 replicate N. crassa frqlv2 luciferase strain with no chemicals added was used as the control.
The plate was then placed in a luminometer in constant darkness for 96 hours to record gene transcription levels through the bioluminescence of luciferase reporter at 30 minute intervals. The luminometer measures in relative light units (RLU) as the system does not translate measurements into units of photons. The RLU’s signifies the level of frq mRNA transcription that occurs. The assay was performed for 96 hours. The luciferase activity reflects the frq mRNA level in the cell. There were six replicates for each strain and 12 replicates of the control without any chemicals.
Results
First, we wanted to test if the selected environmental chemicals affect the levels of the key clock gene frq. Using luciferase-‐bearing N. crassa, we measured the changes of frq levels (see Materials and Methods). The average levels of frq-‐luciferase levels are summarized in Figure 1. The RLU averages are reported as: pseudoephedrine-‐1077.30 RLU, phenylephrine-‐3770.03 RLU, Pall Mall cigarette-‐7289.96 RLU, Natural American Spirit cigarette-‐7700.56 RLU, caffeine-‐380.71 RLU, coffee-‐4692.20 RLU, and the control-‐2686.29 RLU.
All chemical groups show statistically significant differences from the control group (t-‐test p<0.001). To further verify the findings an ANOVA test was applied to the results to determine if a significant result occurred between all groups. All groups had statistically significant difference at a value of p<0.0001 with the exception of both cigarette groups (Pall Mall and Natural American Spirit). When each cigarette group was compared to any other group there appeared to be a significant difference, however, there was no difference in frq-‐luciferase levels between Pall Mall cigarette and the Natural American Spirit cigarette (p=0.39). The comparison was made between cigarettes to find if a difference in ingredients would significantly alter the transcription level of the frq gene. Two other comparisons were made between pseudoephedrine and phenylephrine, and caffeine tablet and coffee. Results showed pseudoephedrine group compared to phenylephrine group and caffeine tablet group compared to coffee group all proved to be statistically significant at values p<0.01.
The phenylephrine group showed to maintain a circadian rhythm with a similar period of the control group. Pseudoephedrine showed a significantly low rhythm with a diminished period (Fig S1). The two cigarette groups were compared along with the control (Fig S2). The Pall Mall cigarette group and the Natural American Spirit cigarette group follow a close circadian rhythm for the first day up until day two. Shortly after the second day the Natural American Spirit group displays an arrhythmic period and in approximately the middle of day two the Pall Mall cigarette group also begins an arrhythmic period. Near the start of the third day both cigarette groups display an inexplicable rhythm. Normally a period occurs once a day, however, for both cigarette groups the period occurs approximately three times on day four. The caffeine group and coffee group were compared along with the control group to reveal contrasting differences in N. crassa’s circadian rhythm. The period displayed by the coffee group matches closely to the control group maintaining a steady rhythmicity (Fig S3). Surprisingly the caffeine group lacked a noticeable rhythm.
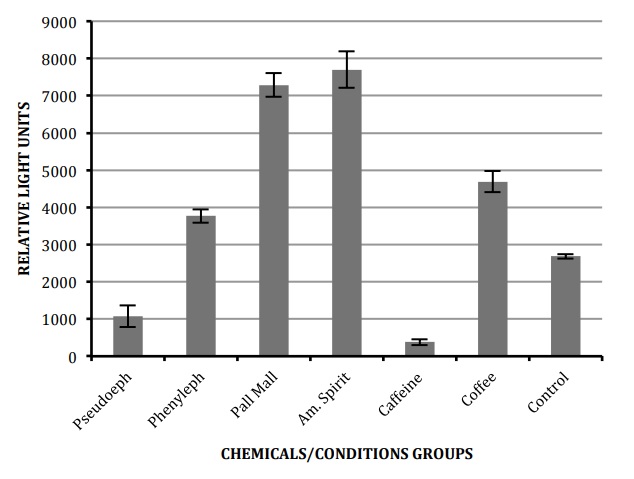
Figure 1. A graph showing the average transcription levels of frq gene as reported by the luciferase reporter. Six replicates from each chemical/condition were averaged in as well as the 12 replicates of the controls strain. The error bar represents standard error.
Discussion
This study demonstrated that the common environmental chemicals used can influence the level and the circadian rhythm of the frq gene, a key molecular component, in N. crassa’s circadian rhythm. The chemicals used in the study are known to have stimulant effects on mammalian physiology. The frq gene being part of the negative feedback loop of N. crassa’s circadian oscillator, normally shows increasing transcription levels from mid-‐day to late day (Baker 2012). Pseudoephedrine and caffeine were able to suppress the frq gene essentially decreasing the negative feedback loop producing no discernible rhythm. Phenylephrine and coffee produced a similar rhythmic pattern to that of the control which could mean the circadian oscillator was able to function correctly without inhibiting either the positive or negative feedback loop. Pseudoephedrine and phenylephrine being entirely different chemicals could explain the drastic change of rhythm they produced. Coffee and caffeine share some of the same properties as caffeine is a chemical within coffee, but coffee contains other chemicals such as monoamine oxidase inhibitors, and β-‐carbolines norharman and harman which may provide an explanation as to the continued rhythmicity of the frq gene. Further tests may be applied with monomine oxidase and β-‐carbolines to determine how frq transcription would react in regards to coffee. The cigarette brands Pall Mall and Natural American Spirit showed very similar effects. With Pall Mall containing additives and Natural American Spirit absent of additives and organically grown, both resulted with no significant difference in RLUs or circadian rhythm. However, it is a curious anomaly as both cigarettes showed arrhythmicity towards the second half of the 96 hour experiment. This severe interference recorded may be due to the stimulant drug, nicotine, or a variety of other chemicals present within cigarettes. As no additives were declared in the Natural American Spirit brand, and both showed similar results, it can be surmised that the results were due to nicotine. Supplementary testing of nicotine alone would need to be applied to compare if a variance in frq transcription or a change in the period of the circadian rhythm occurs to discern if nicotine is an influencing factor. Further investigation would be required to determine how these chemicals interact with other components of N. crassa’s circadian oscillator. Future results of studies of this nature may give more insight on how chemicals, which are commonly used in our daily lives, are affecting the circadian rhythms of other eukaryotic organisms including humans.
Acknowledgements
I’d like to thank Dr. Kwangwon Lee for his patience and guidance every time I came to his office with a dumbfounded look of confusion on my face. I’d also like to thank my wife, Sarah, for her encouragement and support through the late nights/early mornings of study and research. Lastly, I’d like to thank Mike O’Brien for helping me format graphs in excel. The study was performed as part of the course requirement for General Microbiology Laboratory at Rutgers University – Camden.
References
Baker, C. L., J. J. Loros, et al. (2012). “The circadian clock of Neurospora crassa.” FEMS microbiology reviews 36(1): 95-‐110.
Gillies, H., W. E. Derman, et al. (1996). “Pseudoephedrine is without ergogenic effects during prolonged exercise.” J Appl Physiol 81(6): 2611-‐2617.
Herraiz, T. and C. Chaparro (2006). “Human monoamine oxidase enzyme inhibition by coffee and β-‐carbolines norharman and harman isolated from coffee.” Life Sciences 78(8): 795-‐802.
Hodges, K., S. Hancock, et al. (2006). “Pseudoephedrine enhances performance in 1500-‐m runners.” Med
Sci Sports Exerc 38(2): 329-‐333.
O’Neill, J. S., G. van Ooijen, et al. (2011). “Circadian rhythms persist without transcription in a eukaryote.” Nature 469(7331): 554-‐558.
Journal of Biological Sciences at Rutgers Camden (JBS) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
